Does Bt maize expressing Cry1Ac protein have adverse effects on the parasitoid Macrocentrus cingulum (Hymenoptera: Braconidae)?
Abstract
The potential effects of insect-resistant, genetically engineered (GE) crops on non-target organisms, especially on predators and parasitoids, must be evaluated before their commercial cultivation. The effects of GE maize that produces Cry1Ac toxin on the parasitoid Macrocentrus cingulum were assessed by direct bioassay and indirect bioassay. In the indirect bioassay, parasitism rate, cocoon weight and the number of M. cingulum progeny produced per host were significantly reduced when M. cingulum-parasitized Cry1Ac-susceptible Ostrinia furnacalis were fed a diet containing purified Cry1Ac; however, life-table parameters of M. cingulum were not adversely affected when the same assay was performed with Cry1Ac-resistant O. furnacalis. These results indicated that the detrimental effects detected with a Cry1Ac-susceptible host were mediated by poor host quality. In a direct bioassay, no difference in life-table parameters were detected when M. cingulum adults were directly fed a 20% honey solution with or without Cry1Ac; however, survival and longevity were significantly reduced when M. cingulum adults were fed a honey solution containing potassium arsenate, which was used as a positive control. The stability and bioactivity of Cry1Ac toxin in the food sources and Cry1Ac toxin uptake by the host insect and parasitoid were confirmed by enzyme-linked immunosorbent assay and sensitive-insect bioassays. Our results demonstrate that M. cingulum is not sensitive to Cry1Ac toxin at concentrations exceeding those encountered in Bacillus thuringiensis maize fields. This study also demonstrates the power of using resistant hosts when assessing the risk of genetically modified plants on non-target organisms and will be useful for assessing other non-target impacts.
Introduction
Genetically engineered (GE) crops expressing insecticidal Cry toxins derived from Bacillus thuringiensis Berliner (Bt) have been widely planted in many countries. Such GE crops have been effective in controlling insect pests and have benefitted human health and the environment by significantly reducing the need for conventional insecticides (Hellmich et al., 2008).
Maize (Zea mays L.) is one of the most versatile emerging crops having wider adaptability under varied agro-climatic conditions. GE maize varieties expressing Bt Cry toxins are the most widely grown insect-resistant transgenic plants. Bt maize has mainly been designed to protect maize from lepidopteran pests such as corn borers (mainly Ostrinia nubilalis and Ostrinia furnacalis) and some coleopteran pests including Diabrotica virgifera virgifera and Diabrotica barberi (James, 2014). Since its commercial development in 1996, GE maize has been increasingly cultivated and in 2013 was planted on 57.4 million ha, which represented 32% of the worldwide maize cultivation area (Li et al., 2014c).
China has devoted great effort and financial resources to develop GE maize lines and has made significant progress in breeding new varieties of GE maize. Therefore, many new GE maize events and varieties have been extensively evaluated for their resistance to target pests and also for their potential risks to the environment (Romeis et al., 2014). One risk associated with the planting of Bt crops is their potential effects on non-target organisms, especially predators and parasitoids responsible for the biological control of insect pests (Lu et al., 2012). Thus, rigorous environmental risk assessments must be conducted before a novel GE variety is commercially cultivated (Romeis et al., 2013b).
To date, many laboratory, glasshouse, semi-field and field studies have investigated the potential effects of Bt crops on non-target, beneficial species (Romeis et al., 2006). Quite a few adverse effects on life-table parameters of different predators and parasitoids have been reported in studies in which the predators or parasitoids were fed herbivores that were themselves sensitive to Cry toxin and were sub-lethally affected. It thus appears that the adverse effects on a predator or parasitoid might in some cases be explained by reduced prey/host quality rather than by a direct effect of the plant-produced Cry toxin on the predator or parasitoid (Romeis et al., 2006; Naranjo, 2009). Such so-called ‘prey quality-mediated effects’ have been observed in numerous tritrophic systems with Bt-transgenic plants (Alvarez-Alfageme et al., 2009; Li et al., 2013; Li et al., 2014a; Han et al., 2015). Although all of these adverse effects on predators and parasitoids could be indirect, direct effects of Bt could not be excluded. ‘Prey quality-mediated effects’ can be avoided in experiments by using a herbivore that is not susceptible to the plant-expressed Cry toxins (i.e., a non-susceptible herbivore) or by using a strain of the target organism that is highly resistant to the particular toxin (i.e., a Bt-resistant herbivore). The use of a non-susceptible herbivore or a Bt-resistant herbivore ensures that the herbivores themselves are not adversely affected by the ingested toxin (Chen et al., 2008; Lawo et al., 2010; Li et al., 2011; Tian et al., 2014; Wu et al., 2014; Su et al., 2015).
The most important pest of maize in China and Southeast Asia is the Asian corn borer, Ostrinia furnacalis. An insect-resistant GE maize line transformed with the cry1Ac gene provides good resistance against stem borers and was named BT799 (Wang et al., 2014). This GE maize line was independently developed by China Agricultural University and has entered the pilot production stage. The current research concerns the potential effects of this GE maize line on Macrocentrus cingulum (Hymenoptera: Braconidae), a dominant and abundant parasitoid that can suppress corn borers in maize (Dittrick & Chiang, 1982; Cossentine & Lewis, 2011).
M. cingulum is a polyembryonic endoparasitoid of corn borer larvae and is widely distributed in Europe, Japan, Korea and China (Watanabe, 1967). M. cingulum generally completes larval development within a single host; it follows that if its host feeds on Bt plants, the parasitoid larva is likely to become exposed to the Cry toxin (Xu et al., 2007). Because adults of M. cingulum feed primarily on nectar, they also may be exposed to Cry toxins in Bt maize fields (Orr & Pleasants, 1996). To our knowledge, the potential effects of Cry1Ac-producing maize line BT799 on non-target organisms, especially parasitoids, have not been reported. As part of the environmental risk assessment of Bt maize BT799 and other Bt crops, we have selected M. cingulum as a surrogate, non-target species.
In the current study, we developed an experimental system for evaluating the potential effects of insecticidal compounds on M. cingulum larvae by using Bt-susceptible and Cry1Ac-resistant O. furnacalis strains to eliminate potential host-quality mediated effects on this non-target parasitoid. We then used the experimental system to evaluate the indirect effects of Cry1Ac on M. cingulum larvae. In addition, the direct toxicity of purified Cry1Ac toxin to M. cingulum adults was assessed with toxin dosages that were much higher than those likely encountered in the field.
Materials and methods
Insects
Specimens of the parasitoid wasp M. cingulum were collected from O. furnacalis larvae on infested maize stalks at the experimental field station of the Institute of Plant Protection (IPP), the Chinese Academy of Agricultural Sciences (CAAS), near Langfang City, Hebei Province, China (39.5°N, 116.7°E). An M. cingulum colony was subsequently maintained in the laboratory on O. furnacalis larvae. Larvae of O. furnacalis were reared on an artificial diet as described by Zhou et al. (1992) and were maintained in an environmental chamber at 28 ± 1°C, with 70%–80% relative humidity (RH) and a 16 : 8 light : dark cycle (L : D) photoperiod. Adult parasitoids were fed with a 20% honey solution. They were maintained in an environmental chamber at 24 ± 1°C, with 75% ± 5% relative humidity (RH) and with a 16 : 8 L : D. O. furnacalis larvae were exposed to M. cingulum adults as described by Hu et al. (2003).
Two strains of the Asian corn borer, O. furnacalis, were used in this study: a Bt-susceptible strain (ACB-BtS) and a Cry1Ac-resistant strain (ACB-AcR). The ACB-BtS strain has never been exposed to Bt and has been maintained for many years in the laboratory on an artificial diet as described by Zhou et al. (1992). The ACB-AcR strain was selected from the ACB-BtS strain using trypsin-activated Cry1Ac toxin as described by Xu et al. (2015). After 53 generations of selection, the ACB-AcR strain could survive well and complete its development on an artificial diet containing Cry1Ac toxin, and its Cry1Ac resistance was more than 100 times greater than that of the ACB-BtS strain.
Insecticidal compounds
Insecticidal compounds used in the present study included potassium arsenate (PA, KH2AsO4) and the Cry1Ac toxin. PA was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was used as a positive control. The activated, salt-free Cry1Ac toxin was purchased from Envirotest-China (an agent for Envirologix Inc., Portland, ME, USA; www.envirotest-china.com). The toxins were produced and purified at Case Western Reserve University (USA), where the protoxin from Bt strain HD-73 had been expressed as a single-gene product in Escherichia coli. The E. coli-expressed protoxin inclusion bodies were then dissolved and trypsinized before they were isolated and purified by ion exchange high-performance liquid chromatography (HPLC). The pure fraction was then desalted and lyophilized. Purity ranged from 94% to 96%.
The bioactivity of the Cry1Ac toxin was confirmed with a sensitive-insect bioassay. Briefly, ACB-BtS neonate larvae were fed for 7 days with an artificial diet containing a range of Cry toxin concentrations. The EC50 (toxin concentration resulting in 50% weight reduction compared with the control) of Cry1Ac toxin was estimated to be 0.20 μg/mL diet (data not shown).
Host-mediated effects on M. cingulum
Parasitism and development of M. cingulum on untreated O. furnacalis strains
An experiment was performed to determine the parasitism rate of M. cingulum on untreated Cry1Ac-susceptible and -resistant strains of O. furnacalis. Twenty pairs of M. cingulum adult (within 2 days after emergence) were released into an acrylic-framed cage with netted sides (30 × 40 × 40 cm) in the rearing chamber. They were supplied with a 20% honey solution and were allowed to mate for 24 h. Subsequently, 50 third-instar larvae of each strain (ACB-BtS and ACB-AcR) of O. furnacalis were individually placed on chopped maize cobs (20–50 mm diameter, 1–2 mm thickness), and the cob pieces with 50 O. furnacalis larvae were placed in the cage containing 20 pairs of M. cingulum adults. Four replicates (cages) were tested for each O. furnacalis strain. The O. furnacalis larvae were collected from the cages after a 24-h exposure to M. cingulum and were fed an artificial diet in a rearing box (9.0 cm diameter, 7 cm high). Ten days later, each O. furnacalis larva parasitized by M. cingulum was placed in a 5-mL centrifuge tube (one larva per tube). The M. cingulum larvae that emerged from the O. furnacalis larvae were allowed to develop to cocoons in the same centrifuge tube. The number of O. furnacalis parasitized by M. cingulum and the mortality of O. furnacalis in each treatment were recorded. Parasitism rate (%) was calculated by the following equation: the number of parasitized O. furnacalis / (the number of O. furnacalis inoculated − the number of O. furnacalis that died) ×100. The cocoons were randomly selected, and the total number of M. cingulum progeny produced by each O. furnacalis was recorded. Twenty emerged females and males of M. cingulum were randomly selected from each replicate and were individually placed in a cage containing a 20% honey solution. They were examined daily, and their longevities were recorded until all of the M. cingulum adults had died.
Response of Cry1Ac-susceptible and -resistant strains of O. furnacalis to different Cry1Ac concentrations
Bioassays were conducted to determine the highest concentration of Cry1Ac on which the two strains of O. furnacalis could survive. In these bioassays, Cry1Ac-susceptible O. furnacalis larvae were fed an artificial diet containing Cry1Ac at 0, 0.05, 0.1, 0.2, 0.4, 0.8, 1.6 and 3.2 μg/g of diet, and Cry1Ac-resistant O. furnacalis larvae were fed the same diet containing Cry1Ac at 0, 6, 12, 24, 48 and 96 μg/g. For each treatment, 36 third-instar larvae were tested and were individually kept in each well of 12-well bioassay plates containing artificial diet. Larval development and mortality were recorded daily. The sex of each O. furnacalis pupa was determined, and each pupa was weighed with an electronic balance (CPA224S, Sartorius, Goettingen, Germany; d = 0.1 mg) within 12 h after pupation.
Effects of Cry1Ac on M. cingulum
Based on the results obtained with assay described in the previous section, five treatments were used to assess the indirect effects of Cry1Ac on M. cingulum. For three treatments, ACB-BtS larvae were parasitized by M. cingulum and then were transferred to the artificial diet containing 0.0, 0.1 or 1.0 μg of Cry1Ac per g of medium. For two treatments, ACB-AcR larvae were parasitized by M. cingulum and then were transferred to the artificial diet containing 0.0 or 100 μg of Cry1Ac per g of medium. The methods were the same as described earlier except that the artificial diets used to feed the O. furnacalis larvae were replaced every 2 days. In brief, 20 pairs of M. cingulum males and females (within 2 days after emergence) were released into an acrylic-framed cage containing a 20% honey solution and were allowed to mate for 24 h. Fifty third-instar larvae of O. furnacalis of each strain were placed on maize cobs, which were in turn placed in each cage. Each treatment was replicated four times. The experiment was terminated when almost all of the O. furnacalis larvae had pupated (or when host larvae had died) or when M. cingulum had emerged in the negative control. The mortality and parasitism rate of host larvae were recorded. Parasitoid cocoons were weighed. The number of M. cingulum progeny produced from each O. furnacalis was determined.
The stability and bioactivity of Cry1Ac toxin in the food source and in the hemolymph of O. furnacalis larvae were determined as described later in the Methods.
Direct effects of Cry1Ac on M. cingulum adults
Response of M. cingulum adults to PA in the dietary assay
Based on preliminary experiments, PA was selected as a positive control, that is, as a compound that is known to be toxic to M. cingulum and that could be used to determine whether the assay described in the next section was useful for detecting the effects of toxins on M. cingulum adults. A stock solution of PA was diluted with distilled water and mixed with a 20% honey solution to obtain 0, 10, 25, 50, 75 and 100 μg of PA/mL of honey solution. These concentrations were selected based on preliminary experiments. M. cingulum adults were fed the PA-containing honey water, and the experimental system was the same as described in the first section. The experiment was initiated with 20 pairs of M. cingulum per treatment. The mortality of M. cingulum was recorded daily, and the experiment was terminated when the adults in the control died.
Direct feeding assay with Cry1Ac and M. cingulum adults
Twenty pairs of randomly selected M. cingulum males and females were kept in an acrylic-framed cage with one of seven diets: water only; honey solution containing PA at 50 μg/mL; or honey solution containing Cry1Ac at 0, 1, 5, 25 or 125 μg/mL. Each treatment was represented by four replicate cages. The honey solutions were replaced every 2 days. At the start of the experiment, a plate holding one chopped maize cope with 50 third-instar larvae of susceptible O. furnacalis was placed in each cage. The O. furnacalis larvae were removed from the cage after 24 h and were fed an artificial diet in a rearing box (9.0 cm diameter, 7.0 cm high) until they pupated or until M. cingulum emerged. M. cingulum survival and adult longevity were recorded until the M. cingulum died in the control. In addition, cocoons were weighed within 12 h, and the number of M. cingulum progeny produced per O. furnacalis was determined after the adults emerged from the cocoons.
Stability and bioactivity of Cry1Ac toxin in diets, host larvae and adult parasitoids
The temporal stability and bioactivity of Cry1Ac toxin were assessed in the artificial diet fed upon by O. furnacalis larvae and in the hemolymph of O. furnacalis larvae. Diet samples were collected after diets containing 100 μg/g Cry1Ac had been exposed to the parasitized, resistant O. furnacalis larvae for 2 days. Hemolymph samples were collected from parasitized, resistant O. furnacalis larvae that had fed on artificial diet containing 100 μg/g Cry1Ac for 2 days. Both the diet and the hemolymph were represented by three replicate subsamples. The Cry1Ac toxin concentrations and bioactivities in the samples were determined as described in the following sections.
The temporal stability and bioactivity of Cry1Ac toxin were also assessed in two kinds of samples associated with M. cingulum. One kind of sample was collected from honey solution that initially contained 125 μg/g Cry1Ac and that had been used to feed M. cingulum adults for 2 days. The other kind of sample was collected from the body of M. cingulum adults that had fed on that solution for 2 days. Each kind of sample was represented by three replicates. The Cry1Ac toxin concentrations and bioactivities were determined by enzyme-linked immunosorbent assay (ELISA) and by a ‘sensitive-insect’ bioassay as described in the following sections.
ELISA measurements
The concentrations of Cry1Ac in the artificial diet, 20% honey solution, and insects were measured by a double-antibody sandwich ELISA assay, using the Cry1Ac detection kits from Agdia (Elkhart, IN, USA). Before analyses, all insects were washed in phosphate-buffered saline with Tween 20 (PBST) buffer (provided in the kit) to remove any Bt toxin from their outer surface. For Cry1Ac toxin extraction, samples of insects or artificial diets were weighed and mixed with PBST at a ratio of 1 : 10 to 1 : 1000 (mg sample: μL buffer) in 1.5-mL centrifuge tubes. The samples were then fully ground by hand using a plastic pestle. After centrifugation and appropriate dilution of the supernatants (the honey solution samples did not require dilution), ELISA was performed according to the manufacturer's instructions. The measured optical density (OD) values were calibrated to a range of concentrations of Cry1Ac standards made from purified toxin solutions.
Sensitive-insect bioassay
Samples of artificial diets containing 100 μg/g Cry1Ac and honey solution containing 125 μg/g Cry1Ac were prepared that had been exposed to test insects for 2 days. ACB-BtS neonate larvae of O. furnacalis were used as sensitive insects to assess the bioactivity of the Cry1Ac toxins in samples. The samples, which were identical to the supernatants used for ELISA described in the previous section, were incorporated into the artificial diet for O. furnacalis in 24-well bioassay plates. One ACB-BtS neonate larva was added to each well, and each kind of sample containing Cry1Ac was represented by three replicates, with 48 larvae (two plates) per replicate. After 7 days, larval mortality was assessed.
Statistical analyses
In the experiment comparing parasitism of Cry1Ac-susceptible and -resistant strains of O. furnacalis, mortality and parasitism rates of O. furnacalis were compared with Chi-square tests, the numbers of M. cingulum progeny produced per O. furnacalis were compared with Student's t-test, and the longevity of M. cingulum adults was compared with a Mann-Whitney U-test. In other experiments concerning the effects of PA or Cry1Ac toxin on O. furnacalis or M. cingulum, pupal weight or cocoon group weight, and the number of M. cingulum progeny produced per host were compared between each treatment and the negative control (no Cry1Ac added) with Dunnett's test, because the assumptions for parametric analyses (normal distribution of residues and homogeneity of error variances) were met. Because the assumptions for parametric analyses were not met for host mortality, parasitism rate, adult M. cingulum survival, host developmental time, or adult M. cingulum longevity, these data were analyzed by Chi-square and Mann-Whitney U-tests. Bonferroni correction was used for these tests to correct for multiple pair-wise comparisons. The survival of O. furnacalis or M. cingulum in response to different dietary treatments was analyzed using the Kaplan-Meier procedure and Logrank test.
For the indirect bioassay, one-way analyses of variance (ANOVAs) followed by Tukey Honestly Significant Difference (HSD) tests were used to compare the effects of treatments on O. furnacalis mortality, the parasitism rate, cocoon group weight and the number of M. cingulum progeny produced per host.
All statistical analyses were conducted with SPSS software (version 16 for Windows, 2009: SPSS Inc., Chicago, IL, USA).
Results
Parasitism and development of M. cingulum on untreated O. furnacalis
In the absence of Cry1Ac exposure, neither O. furnacalis mortality nor the rate at which O. furnacalis was parasitized by M. cingulum significantly differed between the ACB-BtS and ACB-AcR strain of O. furnacalis (Table 1). More than 80% of the larvae of the two strains were parasitized by M. cingulum, and the number of M. cingulum progeny produced per O. furnacalis larva did not significantly differ between the strains (Table 1). The longevity of M. cingulum adults was also unaffected by O. furnacalis strain (Table 1). These results confirmed that the different genetic backgrounds of the O. furnacalis strains did not significantly affect parasitism of O. furnacalis by M. cingulum.
| Parameter | ACB-BtS | ACB-AcR | Statistics† |
|---|---|---|---|
| O. furnacalis mortality (%) | 6.0 | 5.0 | χ2 = 0.19, P = 0.66 |
| Parasitism rate (%) | 82.5 | 84.2 | χ2 = 0.21, P = 0.65 |
| Number of M. cingulum per larvae (± SE) | 35.8 ± 1.33 | 33.6 ± 1.51 | t = 1.07, P = 0.33 |
| M. cingulum adult longevity (d ± SE) | 7.4 ± 0.25 | 7.7 ± 0.18 | U = -5.50, P = 0.486 |
- †As indicated, statistics are from Chi-square, Student's t-test and Mann-Whitney U-test.
Effects of Cry1Ac on Cry1Ac-susceptible and -resistant strains of O. furnacalis
The survival rate of the ACB-BtS strain steadily declined as the concentration of Cry1Ac in the artificial diet increased, and no larvae survived to the pupal stage at the highest concentration (3.2 μg/g) (Fig. 1); the pupation rate was > 85% in the control. No statistical difference in survival rate was observed between treatments containing Cry1Ac at 0.05 μg/g (χ2 = 0.17, P = 0.68), 0.1 μg/g (χ2 = 0.83, P = 0.36), 0.2 μg/g (χ2 = 1.73, P = 0.19) and 0.0 μg/g (the control). In contrast, survival rates were significantly decreased compared to the control with Cry1Ac at 0.4 μg/g (χ2 = 9.46, P = 0.002), 0.8 μg/g (χ2 = 9.04, P = 0.003), 1.6 μg/g (χ2 = 20.96, P < 0.001) and 3.2 μg/g (χ2 = 34.13, P < 0.001) (Fig. 1). A similar dose-dependent response was found for the other life-table parameters, including third-instar larval development time and female and male pupal weight (Table 2). Male pupal weight appeared to be a more sensitive indicator of toxicity than third-instar larval development time because male pupal weight but not larval developmental time was significantly reduced by Cry1Ac at 0.05 μg/g (Table 2). Based on these results, we selected Cry1Ac concentrations of 0.1 μg/g and 1 μg/g to be used with the susceptible strain in the experiment concerning ‘Host-meditated effects’ (described in the following section); these two concentrations provided a low and moderate level of toxicity, respectively.
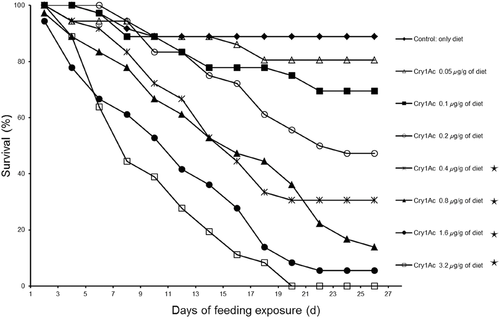
| Weight per pupa (mg ± SE) | |||
|---|---|---|---|
| Cry1Ac concentration (μg/g) | Days to adult ( ± SE) | Female | Male |
| 0.0 | 12.9 ± 0.56 | 67.91 ± 1.74 | 53.16 ± 1.20 |
| 0.05 | 15.2 ± 0.56 | 64.13 ± 2.22 | 50.41 ± 1.23* |
| 0.1 | 15.6 ± 0.68* | 61.98 ± 1.72 | 45.83 ± 1.06* |
| 0.2 | 18.1 ± 1.14* | 54.31 ± 2.54* | 43.03 ± 2.00* |
| 0.4 | 19.0 ± 1.55* | 45.07 ± 2.80* | 40.4 ± 1.29* |
| 0.8 | 25.4 ± 1.81* | 47.15 ± 2.55* | 34.20 ± 0.47* |
| 1.6 | 22.5 ± 2.50* | 43.40 | 33.80 |
| 3.2 | – | – | – |
- Each Cry1Ac treatment was compared to the negative control, which was artificial diet without Cry1Ac. An asterisk indicates a significant difference between a Cry1Ac treatment and the negative control according to the Mann-Whitney U-test (with Bonferroni correction, adjusted α = 0.0083) for developmental time and according to the Dunnett's test for pupal weight.
- “–” indicates that no larvae survived the observation period.
The survival rate of the ACB-AcR strain, which was > 90% in all cases, decreased slightly as the concentration of Cry1Ac toxin increased but the decrease was not statistically significant (P > 0.05) according to pairwise comparisons (Fig. 2). Similarly, third-instar larval development time and female and male pupal weight of ACB-AcR were not significantly affected (P > 0.01) by the concentration of Cry1Ac in the artificial medium (Table 3). Based on these results, we selected a Cry1Ac concentration of 100 μg/g to be used with the resistant strain in the experiment concerning ‘Host-meditated effects’ (described in the following section).
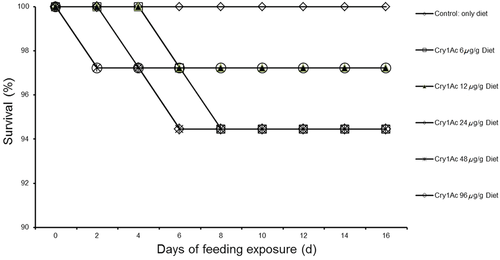
| Weight per pupa (mg ± SE) | |||
|---|---|---|---|
| Cry1Ac concentration (μg/g) | Days to adult ( ± SE) | Female | Male |
| 0.0 | 10.8 ± 0.39 | 70.74 ± 1.42 | 47.40 ± 1.39 |
| 6.0 | 10.4 ± 0.30 | 71.25 ± 1.60 | 50.49 ± 1.83 |
| 12.0 | 10.4 ± 0.28 | 71.11 ± 1.46 | 52.21 ± 1.68 |
| 24.0 | 10.2 ± 0.29 | 70.84 ± 1.29 | 52.55 ± 1.82 |
| 48.0 | 10.1 ± 0.28 | 74.08 ± 1.10 | 50.53 ± 1.48 |
| 96.0 | 10.3 ± 0.28 | 73.28 ± 0.88 | 47.89 ± 1.66 |
- Each Cry1Ac treatment was compared to the negative control, which was artificial diet without Cry1Ac. No significant difference was detected between any Cry1Ac treatment and the negative control according to the Mann-Whitney U-test (with Bonferroni correction, adjusted α = 0.01) for developmental time and according to the Dunnett's test for pupal weight.
Host-mediated effects of Cry1Ac on M. cingulum
The mortality of ACB-BtS larvae was higher (P < 0.001) when ACB-BtS larvae that had been exposed to M. cingulum were fed artificial diets containing 0.1 and 1 μg/g Cry1Ac rather than 0.0 μg/g Cry1Ac, and mortality was higher with 1 μg/g than with 0.1 μg/g Cry1Ac (P < 0.001) (Table 4). Only a few M. cingulum-parasitized ACB-BtS larvae pupated at 1 μg/g Cry1Ac. The rate at which M. cingulum parasitized ACB-BtS larvae was significantly lower (P = 0.006) with 1 μg/g than with 0.0 μg/g Cry1Ac and was intermediate with 0.1 μg/g (Table 4); no parasitism occurred with 1 μg/g Cry1Ac. The total weight of cocoons produced per parasitized ACB-BtS larva was significantly lower (P < 0.05) with 0.1 μg/g Cry1Ac than with 0.0 μg/g Cry1Ac (Table 4). The number of M. cingulum progeny produced per parasitized ACB-BtS larva was significantly lower (P < 0.05) with 0.1 μg/g Cry1Ac than with 0.0 μg/g Cry1Ac (Table 4). For M. cingulum-parasitized ACB-AcR larvae, mortality, M. cingulum parasitism rate, total weight of cocoons produced per larva and the number of M. cingulum progeny produced per larva did not significantly differ on diets containing 0.0 μg/g and 100 μg/g Cry1Ac (Table 4).
| O. furnacalis strain | Cry1Ac concentration in the diet (μg/g) | O. furnacalis mortality (%) | Parasitism rate (%) | Total weight of cocoons produced per host (mg ± SE) | Number of M. cingulum emerged per host (± SE) |
|---|---|---|---|---|---|
| ACB-BtS | 0.0 | 7.5 ± 2.22 a | 32.7 ± 6.57 a | 90.48 ± 1.88 a | 38.8 ± 1.28a |
| ACB-AcR | 0.0 | 5.0 ± 1.29 a | 38.0 ± 4.80 a | 98.07 ± 1.62 a | 35.9 ± 0.71a |
| ACB-BtS | 0.1 | 35.0 ± 3.41 b | 15.2 ± 6.09 ab | 75.90 ± 2.64 b | 21.5 ± 0.31b |
| ACB-BtS | 1.0 | 92.5 ± 4.35 c | 0.0 ± 0.0 b | – | – |
| ACB-AcR | 100 | 7.5 ± 2.22 a | 35.3 ± 7.16 a | 89.96 ± 2.38 a | 37.3 ± 1.16a |
- “–” indicates that no larvae survived.
- Means in a column followed by different letters are significantly different (one-way analyses of variance with Tukey tests).
The stability and bioactivity of Cry1Ac were determined using a diet containing 100 μg/g Cry1Ac that was fed on by nonparasitized Cry1Ac-resistant larvae of O. furnacalis. First, the ability of ELISA to detect Cry1Ac in the artificial diet was determined using fresh diet that had not been exposed to larvae and that contained 100 μg/g Cry1Ac; the detection efficiency was 51.2%, that is, ELISA detected 51.2 ± 6.51 μg (mean ± SE) of Cry1Ac per g of artificial diet. After a 2-day feeding exposure, the mean Cry1Ac concentration in the diet declined to 36.1 ± 3.59 μg/g, which was significantly lower than the concentration in the fresh diet (t = 3.52, df = 4, P = 0.024).
Because M. cingulum larvae grow and develop in the larval hemolymph, we used ELISA to determine the Cry1Ac concentration in the hemolymph of Cry1Ac-resistant O. furnacalis larvae that had fed on diet containing 100 μg/g Cry1Ac. The concentrations of Cry1Ac detected in the hemolymph ranged from 203.2 to 557.8 ng/mL with a mean of 360.7 ng/mL. No Cry toxin was detected in O. furnacalis larvae that had been fed on artificial diet without Cry1Ac.
In the sensitive-insect bioassays, the mortality of ACB-BtS neonate larvae feeding on an artificial diet containing Cry1Ac extracted from the diet that initially contained 100 μg/g Cry1Ac for 7 days was 45.1%, which was much higher than the 6.3% mortality in the negative control.
All of these results confirm that Cry1Ac toxin in the artificial diet and in the hemolymph of O. furnacalis larvae that fed on that diet had substantial bioactivity and that the bioactivity did not decline significantly during the 2-day feeding exposure to O. furnacalis.
Assay validation
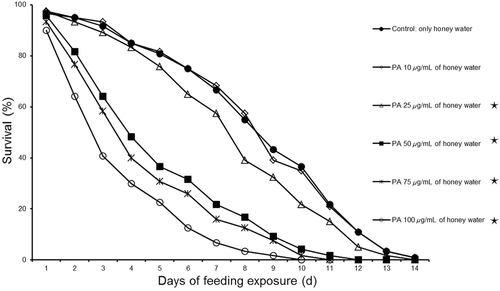
An experiment was conducted with PA to determine whether the assay system was suitable for detecting direct toxic effects of Cry1Ac on M. cingulum adults and to select a PA concentration to be used as a positive control in the assay with Cry1Ac. As the concentration of PA in the diet increased, the survival rate of adult M. cingulum declined, and no adult survived after 10 days at the highest PA concentration (Table 5, Fig. 3). A similar dose-dependent response was evident for the longevity of M. cingulum adults (Table 5). These results indicated that the assay system was suitable for detecting direct toxic effects on M. cingulum adults. Based on the results, 50 μg/mL PA was selected as the positive control for the direct feeding bioassay with M. cingulum adults (see next section).
| PA concentration | Adult | Adult longevity |
|---|---|---|
| (μg/mL) | survival (%) | longevity (days ± SE) |
| 0 (control) | 36.1 | 8.6 ± 0.30 |
| 10 | 34.5 | 8.6 ± 0.29 |
| 25 | 21.7 | 7.8 ± 0.29 |
| 50 | 4.2* | 5.1 ± 0.26* |
| 75 | 1.7* | 4.6 ± 0.24* |
| 100 | 0.0* | 3.7 ± 0.20* |
- An asterisk indicates a significant difference between a PA treatment (≥ 10 μg/mL) and the control (0 μg/mL) according to a Chi-square test with Bonferroni corrections (adjusted α = 0.01) for adult survival and according to a Mann-Whitney U-test with Bonferroni correction (adjusted α = 0.01) for adult longevity.
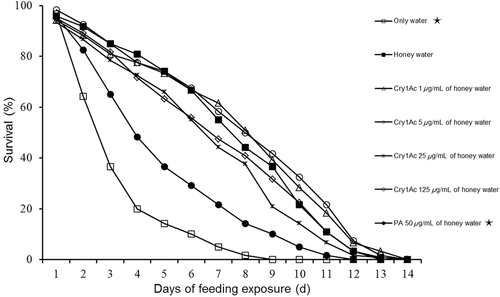
Direct feeding assay with Cry1Ac and M. cingulum adults
Adult M. cingulum survival did not significantly differ (χ2 test; P > 0.05) between any Cry1Ac treatment (honey solution with ≥ 1 μg/mL Cry1Ac) and the negative control (honey solution without Cry1Ac) (Fig. 4). However, survival was significantly lower (P < 0.001) with pure water or with honey solution containing PA than with the negative control. No differences were found between any Cry1Ac treatment (honey solution with ≥ 1 μg/mL Cry1Ac) and the negative control for the following parameters: adult longevity (P > 0.0083), total weight of cocoons produced per host, and the number of M. cingulum progeny produced per host (P > 0.05) (Table 6). Adult longevity and the number of M. cingulum progeny produced per host were significantly lower (Dunnett's test; P < 0.05) with honey solution containing PA than with pure honey solution (Table 6).
| Adult longevity | Total weight of cocoons | Number of M. cingulum | |
|---|---|---|---|
| Treatment | |||
| (days ± SE) | produced per host (mg ± SE) | progeny per host (± SE) | |
| Pure water | 3.5 ± 0.17* | 93.46 ± 4.56 | 34.9 ± 2.14 |
| Pure honey solution | 8.2 ± 0.36 | 101.52 ± 4.31 | 39.7 ± 2.70 |
| Cry1Ac at 1 μg/mL | 8.5 ± 0.39 | 97.65 ± 5.05 | 41.0 ± 3.06 |
| Cry1Ac at 5 μg/mL | 7.4 ± 0.35 | 96.14 ± 4.66 | 38.0 ± 2.33 |
| Cry1Ac at 25 μg/mL | 7.1 ± 0.35 | 106.98 ± 4.50 | 42.7 ± 2.16 |
| Cry1Ac at 125 μg/mL | 8.8 ± 0.39 | 103.86 ± 3.22 | 37.5 ± 2.20 |
| PA at 50 μg/mL | 5.1 ± 0.26* | 91.72 ± 4.42 | 29.4 ± 1.58* |
- Pure honey solution served as a negative control, and honey solution containing PA served as a positive control. An asterisk indicates a significant difference between the PA treatment or a Cry1Ac treatment and the negative control according to a Mann-Whitney U-test with Bonferroni correction (adjusted α = 0.0083) for adult longevity and according to a Dunnett's test for total weight of cocoons produced per host and progeny number.
We determined the stability and bioactivity of Cry1Ac in one of the diets, that is, in the honey solution that contained 125 μg/mL Cry1Ac and in the M. cingulum adults that fed on that diet. The honey solution was appropriately diluted and then subjected to ELISA. Before the assay started, the honey solution contained 118.8 ± 2.85 μg/mL (mean ± SE) of Cry1Ac toxin. After 2 days of feeding exposure, the Cry1Ac concentration had significantly decreased to 103.0 ± 2.95 μg/mL honey solution (t = 6.68, df = 4, P = 0.003).
After M. cingulum adults had fed for 2 days on a honey solution containing 125 μg/mL Cry1Ac, ELISA indicated that each adult contained from 0.46 to 1.87 μg/g Cry1Ac with a mean of 1.19 μg/g.
In the sensitive-insect bioassay, the mortality of ACB-BtS larvae that fed on a diet supplemented with honey solution that initially contained 125 μg/mL Cry1Ac and that had been used for 7 days in the direct feeding bioassay was 86.8%. This percentage of mortality was substantially higher than the 4.2% mortality in the control.
These results indicate that the M. cingulum adults in the direct feeding assay were exposed to active toxin.
Discussion
One concern with the deployment of Bt crops is their potentially detrimental effects on non-target organisms, in particular their potential effects on predators, parasitoids and other natural enemies that function as biological control agents. Although the majority of studies have shown negligible effects of Bt crops on natural enemies (Romeis et al., 2006; Li et al., 2008; Li et al., 2014a; Li et al., 2014b; Zhang et al., 2014; Wang et al., 2015), negative effects of Bt toxins on non-target parasitoids have been reported in some laboratory and field studies (Baur & Boethel, 2003; Meissle, 2006). Unlike predators, which usually feed on several different species of insects during their lifetime, parasitoid larvae generally complete their development within a single host. Thus, if that host feeds on Bt plants, the parasitoid would be exposed to the Cry toxin. To test the effect of Cry toxin on parasitoids, researchers commonly conduct indirect bioassays in which Cry toxins are delivered to the parasitoid indirectly through their hosts. Researchers also commonly conduct direct feeding bioassays, in which purified Cry toxins are added to artificial diets that are fed to the parasitoid (Alvarez-Alfageme et al., 2011; Li et al., 2011; Romeis et al., 2011). To avoid prey- or host quality-mediated effects in tritrophic experiments, researchers must ensure that the herbivores themselves are not adversely affected by the ingested toxin (Romeis et al., 2006; Naranjo, 2009; Shelton et al., 2009).
To avoid host quality mediated-effects, we used a Cry1Ac-resistant strain of O. furnacalis (ACB-AcR) in this study. First, we determined that the different genetic backgrounds of the two O. furnacalis strains did not discernibly alter the success of M. cingulum parasitization. Some previous study had found that sub-lethal (to the host) concentrations of Cry greatly affect a parasitoid (Walker et al., 2007), therefore, we identified sub-lethal concentrations of Cry1Ac for our study. Based on the results of the bioassay with susceptible and resistant O. furnacalis larvae and with different concentrations of Cry1Ac, we selected 0.1 μg/g and 1 μg/g of Cry1Ac as the sub-lethal concentrations that differ in their effects on susceptible O. furnacalis larvae and that could be used to determine whether the assay was useful for detecting the effects of Cry1Ac on M. cingulum. In the current study, we reported substantial host quality-mediated effects of Cry1Ac on M. cingulum when a Cry1Ac-susceptible strain of O. furnacalis was used. To eliminate host quality-mediated effects, we selected 100 μg/g Cry1Ac as the test concentration and used a Cry1Ac-resistant strain of O. furnacalis. Because this Cry toxin concentration is more than 10 times higher than the concentration likely to occur in Bt plant tissue in the field, it represents a worst-case exposure scenario. When the Cry1Ac-resistant strain of O. furnacalis was used in the current study, this high Cry1Ac toxin concentration did not detrimentally affect any of the M. cingulum life-table parameters that were measured. These findings revealed that Cry1Ac had no detrimental effects on M. cingulum when host quality-mediated effects were eliminated. It is consistent with other studies that evaluated the potential effects of Bt crops on parasitic wasps (Walker et al., 2007; Chen et al., 2008; Raybould et al., 2013; Han et al., 2015,).
When researchers measure the hazard of an insecticidal compound to a non-target organism in a dietary exposure assay, they must confirm the concentration, stability and bioactivity of the test compound in the food source, and also confirm the uptake of the compound by the test organism (Romeis et al., 2011; Raybould et al., 2013; Romeis et al., 2013a; Li et al., 2014d). In our study, ELISA measurements showed that the Cry1Ac toxin concentrations in an artificial diet were relatively stable, with 29.6% degradation occurring during the 2-day feeding exposure. Despite this degradation, the Cry1Ac toxin concentrations in the diets were still higher than the concentration likely to be encountered by O. furnacalis in the field. In addition, our ELISA analysis detected the Cry1Ac toxin in the hemolymph of O. furnacalis larvae where M. cingulum larvae grew and developed. This confirmed that M. cingulum was exposed to Cry1Ac toxin during the bioassay. To clarify whether Cry1Ac toxin retained bioactivity during the 2-day feeding exposure, we performed a sensitive-insect bioassay with O. furnacalis neonate larvae. The results confirmed that the Cry1Ac toxin in the artificial diet was still toxic at the end of the 2-day feeding exposure and therefore that M. cingulum was exposed to high concentrations of bioactive Cry1Ac toxin in the feeding assay.
As noted, the results obtained with Bt-resistant and -susceptible O. furnacalis indicate that the negative effects that occurred when M. cingulum fed on Bt-susceptible O. furnacalis were mediated by host quality. While the negative effects on M. cingulum were probably caused by toxicity to the host larvae resulting from their consumption of Cry1Ac toxin, they might also have been caused by other unknown nutritional differences between the Bt-resistant and -susceptible O. furnacalis. Clarifying the nature of prey and host quality-mediated effects requires research on the immune and metabolic status of Bt-susceptible prey or hosts that have fed on Bt crops (Rahman et al., 2004; Ma et al., 2005; Raybould, 2007; Ericsson et al., 2009). A recent study reported changes in the sugar concentration and composition in larvae of a Cry1Ac-susceptible strain of Helicoverpa armigera (Hübner) and a reduced glycogen content in larvae of a Cry1Ac-resistant strain when fed Bt (Cry1Ac) cotton (Lawo et al., 2010). Further studies on these systems should increase our understanding of the factors that determine the quality of herbivores as prey or hosts in Bt cropping systems.
Although lepidopteran-active Bt toxins are generally considered to lack direct toxicity to parasitoids or predators (Glare & O'Callaghan, 2000), confirming this is difficult. Direct feeding and choice experiments with adult parasitoids, including Cotesia plutellae (Chilcutt & Tabashnik, 1999; Schuler et al., 2004), revealed no harm to the parasitoid. Adults of M. cingulum are not predacious, but they are directly exposed to insecticidal toxin in Bt crops when they forage nectar from plants expressing Cry toxin. In the current study, we conducted a direct feeding bioassay using activated and purified Cry1Ac toxin that was incorporated into a 20% honey solution. Because a credible feeding bioassay should detect any toxicity of test compounds (Duan et al., 2010; Li et al., 2011; Romeis et al., 2011), the known insecticidal compound PA was included in the bioassay. Dose-dependent responses to PA were observed for important life-table parameters, including survival and adult longevity. These results demonstrated: that the assay system developed in the current study was capable of detecting dietary effects of insecticidal compounds; that the honey solution was a reliable medium for transfer of insecticidal compounds to M. cingulum adults; and that survival and adult longevity were sensitive indicators of toxicity. A series of concentrations higher than those measured in maize pollen (unpublished) was used to create a worst-case exposure scenario for the risk assessment (Garcia-Alonso et al., 2006; Romeis et al., 2008). No adverse effect of the Cry1Ac toxin was found on any of the measured M. cingulum life-table parameters. Feeding on diets containing PA, in contrast, significantly reduced the survival and longevity of M. cingulum adults. This positive control demonstrated that our experimental system was able to detect adverse effects and appeared to be appropriate for assessing the effects of toxic compounds on M. cingulum adults.
ELISA measurements confirmed the uptake of Cry1Ac by M. cingulum adults feeding on the honey solution and also confirmed the stability of Cry1Ac toxin in the 2-day feeding exposure. The bioactivity of the Cry1Ac toxin during the feeding exposure was confirmed by a sensitive-insect bioassay. These data indicated that M. cingulum was exposed to high levels of biologically active Cry1Ac toxin in the honey solution. We therefore conclude that M. cingulum adults are not sensitive to Cry1Ac even at high concentrations.
In conclusion, an indirect bioassay in which Cry1Ac toxin was transferred to M. cingulum indirectly through the larvae of O. furnacalis and a direct feeding assay in which adults were directly exposed to a high dose of Cry1Ac toxin were conducted. The results revealed that M. cingulum is not sensitive to Cry1Ac toxin and GE maize containing Cry1Ac have no risk to this non-target organism. The methods developed in the current study will be useful for assessing other non-target impacts of genetically modified crops.
Acknowledgments
This study was supported by grants from the Genetically Modified Organisms Breeding Major Projects (2014ZX08011-003) and the Special Fund for Agro-scientific Research in the Public Interest (201303026).
Disclosure
All authors disclose no potential conflicts of interests, including specific financial interests and relationships and affiliations relevant to the subject of our manuscript.




