Advances in clinical brain–computer interfaces for assistive substitution and rehabilitation: A rapid scoping review
Abstract
Objective
This scoping review explores the rapidly evolving field of brain–computer interface (BCI) technologies, with a particular emphasis on the fundamental concepts, advances made, and prospective applications. Following the 2022 Annual Scientific Meeting of the Hong Kong Neurosurgical Society themed ‘Neuromodulation and Brain-Computer Interface’, this article represents the first comprehensive review of BCI technologies from a Hong Kong perspective, providing a balanced viewpoint that reflects both academic and practical clinical insights for surgeons considering the implementation of these emerging technologies.
Methodology
A rapid scoping review was conducted to clarify key concepts and trends in current BCI technologies, including signal acquisition methods, effectors, applications, and ethical issues. Key developments, particularly those relevant to Hong Kong, were identified and analysed.
Results
We summarise various modalities for the acquisition of central nervous system signals, introducing the techniques and steps involved in the data processing pipeline. We highlight two major arms of BCI applications and their promises in advancing patient care: assistive communication or substitution and closed-loop rehabilitation or neuromodulation. The exciting frontier also invites a host of ethical questions which must be thoroughly discussed.
Conclusions
The growing body of knowledge in BCIs offers new treatment options for patients requiring assistive substitution and rehabilitation. The review hopes to provide a rigorous foundation for future research, invite subsequent discussions and translational studies, and support the incorporation of BCI technologies into local healthcare.
1 INTRODUCTION
The nervous system of human beings interacts with the world and the human body itself. The afferent pathway is the sensory component that detects stimuli from the environment or the internal changes of the body, while the efferent pathway responds to the stimuli or changes, by means of muscle control for locomotion, or endocrine and symptomatic systems.1
A brain–computer interface (BCI) refers to a computer system that quantifies or qualifies the activities of the central nervous system and hence translates them to signals that replace, restore, enhance, supplement, or improve the natural central nervous system outputs.2 The history of BCIs could be dated back to the 1970s at the University of California, Los Angeles.3 The concept of BCIs was proposed by Jacques Vidal in the 1970s in his article named ‘Toward Direct Brain-Computer Communication’.4 In the later part of the article, the author described the pilot project ‘Brain–Computer Interface’ by collecting the electroencephalography (EEG) signals with subsequent computer processing and analysis. In 1988, Farwell and Donchin implemented the P300 component of the event-related potentials for letter choice, aiming at providing the means of communication for patients with locked-in syndrome.5 Pfurtscheller and his team6 first quantified the event-related desynchronisation in 1977 and later applied it as the afferent side of a BCI system, which has provided the basis for inputs such as motor imagery (MI).
In the 1990s, human brain implants were made feasible and BCIs started gaining popularity. In 2011, Kennedy et al7 published their experience with 5 patients who received BCI implants since 1996. Since then, there has been an increasing number of publications on BCIs. In 2012, Hochberg et al8 described their experiences in which two patients with tetraplegia succeeded in reaching and grasping with the help of signals decoded from a local population of neurons in the dominant motor hand area (M1), recorded from a 4 × 4mm 96-channel microelectrode array (MEA). The field has rapidly flourished in the following years, including BCIs for restoring communication by decoding articulatory cortical activity into multiple output modalities in real time achieved by Edward Chang's group,9, 10 and adaptive deep brain stimulation (aDBS) achieved by Simon Little and Philip Starr's group, which dynamically responds to changing clinical and neural states in patients with Parkinson disease (PD), thus addressing residual motor fluctuations in patients previously on conventional DBS.11
2 METHODS
A scoping literature review was conducted to explore the current landscape of BCI technologies, focusing on advances and prospective clinical applications. To address our aim, we formulated four research questions: What are the key steps involved in creating a BCI? How are neural signals processed and decoded? What effectors and applications of BCIs are clinically trialled? What ethical considerations are discussed regarding clinical applications of BCIs? The review aimed to enable a mapping of the ‘extent, range, and nature of research activity’ in this emerging area of research.
2.1 Literature search strategy
Relevant literature was identified through electronic database searches in PubMed, IEEE Xplore, Scopus, and Google Scholar, covering the period up to September 2023. Keywords used in the search included ‘brain–computer interface’, ‘BCI’, ‘neuroprosthetics’, ‘neural signal processing’, ‘neurorehabilitation’, ‘neuromodulation’, and ‘closed-loop stimulation’. Articles were also identified by manually reviewing the reference lists of relevant publications.
2.2 Inclusion and exclusion criteria
To map the breadth and trends of BCI literature, we included peer-reviewed studies irrespective of source, including primary research, reviews, and nonempirical evidence published in English. Articles were included if they discussed BCI technologies, signal acquisition methods, data processing techniques, BCI applications in assistive communication or rehabilitation, or ethical issues related to BCIs. A particular emphasis was placed on recent advances in BCI systems, especially those involving advanced feature selection and neural decoding algorithms, as well as clinical trials of adaptive neuromodulation. Studies including local research contributions from Hong Kong were highlighted. Given the scoping nature of the review, studies were selected based on their relevance and contribution to the understanding of BCI technologies and applications.
2.3 Data extraction and synthesis
Data from the selected articles were extracted and organised into thematic areas corresponding to the sections of the review: signal acquisition methods, signal processing and enhancement, effectors and applications, and ethical considerations. Timelines of key developments, findings, and advancements were synthesised to provide an informed discussion of current and prospective applications of BCIs.
3 SIGNAL ACQUISITION
Broadly, signal acquisition could be divided into invasive, non-invasive, and hybrid techniques. Invasive techniques involve implanting electrodes directly into the cerebral cortex, while non-invasive techniques pick up signals from outside the scalp.
3.1 Non-invasive modalities
One of the most common non-invasive techniques is EEG. While having reasonable temporal resolution, its spatial resolution is limited by poor signal attenuation through physical barriers of the CSF, skull, and scalp.2 EEG recordings are also susceptible to mechanical, electromyographic, and electrooculographic artefacts.12
Magnetoencephalography (MEG) records post-synaptic activity with magnetic fields; functional magnetic resonance imaging detects oxygenation within brain regions as a surrogate marker for neuronal activity with magnetic fields; near-infrared spectroscopy detects oxygenation by photon activity. Non-invasive techniques are relatively cheap and have fewer risks to the patients, but suffer from low signal specificity, low signal-to-noise ratio, and signal distortion.13
3.2 Invasive modalities
Neuroprostheses may be implanted surgically to obtain neural data from the central or peripheral nervous system. With surgical risks come more sensitive and specific data (Figure 1).
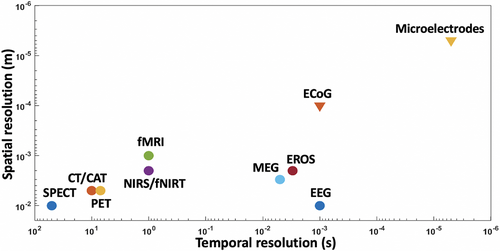
Electrocorticography (ECoG) describes signals acquired from electrodes placed either subdurally or epidurally.14 Local field potentials are detected as summative neuronal processes surrounding the recording electrode. An example of the robustness of ECoG technologies can be found during the 2022 Annual Scientific Meeting of the Hong Kong Neurosurgical Society, where Professor Chang9 from UC San Francisco introduced his work in speech rehabilitation, allowing speech to be decoded in real time with local field potentials acquired from ECoG electrodes implanted across temporal and frontal cortices. Signal activity down to the individual neuron could be recorded using microelectrodes. The smaller the calibre of the electrode, the higher the selectivity, but also the higher the impedance. Microelectrodes at diameters of less than 100 μm are commonly found to be optimal.15
The first microelectrodes were conductive microwires insulated except at the tip. As they only record activity at the exposed tip, increasing the number of recording sites would require increasing the number of electrodes, resulting in a linear increase in probe size and thus neural trauma and gliosis. Silicon-based probes were created as a solution to increase the number of recording sites without increasing the overall probe size. Metal is melded through silicon coating with patterns that expose the metal to form recording sites, read-out pads for connecting to external circuitry, and interconnecting traces between the recording sites and read-out pads.16
Such rigid interfaces, however, suffer from gradual recording quality degradation, which is thought to arise from tissue damage and the ensuing immune response. Softer and more flexible neural probes could be fabricated in a polymeric fashion to mitigate the textural mismatch between probe and brain. Temporary stiffness that may be required during implantation could be imparted by additional surgical shuttles or biodegradable coatings.17, 18
Although sensitive, the quality of signals acquired from more invasive techniques may be affected by foreign body response. The blood–brain barrier and absence of immune cells within the central nervous system mean that it responds differently against foreign bodies. It is thought that the disruption of blood–brain barrier induces reactive gliosis, where microglia and astrocytes activate and encapsulate said foreign body. With time, the electrode may be subject to buckling, corrosion, and degradation, while the brain may suffer from injury due to micromotion of the electrodes.13 Hybrid systems could potentially incorporate the strengths of different technologies while minimising their drawbacks. Different signal inputs from the brain could be integrated simultaneously or sequentially, while signals may be extracted from even outside the brain in neurovisceral circuitry.
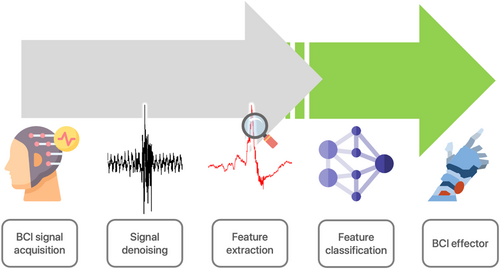
4 SIGNAL PRE-PROCESSING AND SIGNAL ENHANCEMENT
With as many as 100 billion neurons in the brain, cortical signals measured are inundated with noise which must be cleaned, or denoised, before analysis.19, 20 Signal processing usually begins with the removal of various artefacts and noise, after which signals are normalised or standardised. This stage is commonly referred to as pre-processing. Then, valuable features are extracted via different methods introduced below, and fed into classifiers which classify or label signal features corresponding to neurophysiology (Figure 2). The classification performance is quantified and observed. If satisfactory performance is obtained from a model, the model can then be used for further testing using a different independent data set held back from the training data. Holding back testing data from training data ensures unbiased evaluation of the model onto unseen data, although the ability to generalise to all signals cannot be guaranteed by a single testing data set.
4.1 Sources of noise
EEG signals collected from the surface of the scalp are highly contaminated with various types of internal and external artefacts and background noise. The recording unit, including amplifiers and cables, and subject actions, including breathing and blinking, contribute towards disturbances of EEG data.21, 22 EEG data are particularly prone to such disturbances because of their very small amplitude.23
Myopotentials caused by muscle contraction around the temple and forehead are the most common physiological interferences in EEG.24 These artefacts, including extraocular and tongue movements, are also among the most easily identified in EEG recordings due to their expressive amplitudes compared with neuronal potentials and distinctive shape. Extraocular movements generate an alternating current with a high amplitude which is visible on electrodes around the eyes. Unique patterns, meanwhile, are often observed in movement-related disorders (eg, rhythmic sinusoidal artefacts of 4–6 Hz for PD).25 EEG and MEG signals can also encounter strong power line interference at 50 or 60 Hz.26 Although shielded rooms can theoretically reduce the impact of interference on recordings, the mobility of EEG systems in clinical BCI systems makes effective shielding impractical .27, 28
4.2 Denoising techniques
Without proper noise removal techniques, brain signals may be completely suppressed and illegible for further qualitative or quantitative analysis.29 The field of denoising techniques designed for either single- or multi-channel data is rather mature.30, 31 The major techniques are adaptive filters, wavelet transform (WT), or independent component analysis (ICA), the characteristics of which are briefly summarised in Table 1. Certain techniques, including empirical mode decomposition and time–frequency (T–F) image dimensionality reduction, are excluded from further discussion as they cannot be implemented in real time, which heavily restricts their feasibility within BCI systems.32, 33
| Techniques | Additional reference needed | Effectiveness | Computational cost |
|---|---|---|---|
| Regression | Yes | Low | Low |
| Filtering | Depends | Low | Low |
| Wavelet transform | No | Medium | Medium |
| Independent component analysis | No | Medium | Medium |
| Canonical component analysis | No | Medium | Medium |
| Neural networks | No | High | High |
| Hybrid | No | High | High |
Traditional regression methods have been in use for many years. Regression analysis first defines the amplitude relation between a reference channel and the channel of interest, then subtracts estimated artefacts from the raw signal. This algorithm thus requires exogenous reference channels (ie, electrooculography, electrocardiogram) to omit different artefacts. Mutual contamination between the signal and the reference channel, however, may reduce demising effectiveness.34
Often, reference signals are lacking, and the involved neurophysiological processes behind artefacts are complex. Denoising can therefore be considered a blind source separation (BSS) problem, where the original sources underlying the signals are approximated, without a priori knowledge about the physiological sources themselves, and without knowledge about the mixing of such sources. Four main techniques have emerged to specifically tackle the difficult problem of denoising in a BSS context, including WT, ICA, canonical component analysis (CCA), and empirical mode decomposition.
WT was conceptualised in the early 1980s and is performed by deconstructing a signal into the time and frequency domains. Thresholding is then applied to discard the artefacts from the raw signal, where the frequencies of artefacts from experience or from literature are used as the threshold. The remaining data are subsequently added to reconstruct the clean signal.35
The concept of ICA was introduced in 1986 especially to solve the BSS problem.36 ICA operates under three assumptions: that source signals exhibit statistical independence and are instantaneously mixed; the observed signal must be equal to or greater than that of the source signal37; and that sources are either non-Gaussian or only one source is Gaussian. That the true signal is assumed to be mixed with signal artefacts at each time point independently allows the observed signal to be decomposed into independent components. In general, artefacts such as eye movements, eye blinks, and cardio activities originate from mutually independent sources, and volume conduction is linear, rendering the first assumption reasonable. However, physiological signals in the brain are not mixed and propagated instantaneously in a linear fashion; therefore, a convolutive ICA approach, which takes into account weighted and delayed contributions of signals, has also been proposed.38
CCA, by contrast, describes a method to measure the linear relationship between two multi-dimensional random variables.39 The use of CCA in removing muscle artefacts was first described in 2006, where it outperformed low-pass filtering and ICA techniques.40 CCA further differs from ICA in that the former brings shorter computational time compared with the use of higher-order statistics in ICA.
In recent years, neural networks have been increasingly adopted in denoising due to their real-time applicability and high accuracy. One of the first deep convolutional auto-encoders designed to eliminate ocular and jaw-clenching artefacts demonstrated superiority over traditional filtering methods.41 The dedicated removal of ocular artefacts using deep learning was further explored in Yang et al.42 Further research demonstrated promise for neural networks to remove muscular and cardiac artefacts. Neural plasticity as a result of pathological events such as epilepsy, drug alterations, rehabilitation regimens, and biological cycles (eg, circadian rhythms) might alter the dynamics of the brain and require adaptive retraining of neural networks before generalising to unseen signals.43, 44
Hybrid methods have also been proposed, offering better performance at the cost of increased computational complexity. Several studies combined WT and ICA to separate artefact-independent components, resulting in clean data within improved computational times.45 For removing ocular artefacts, the combination of WT-based and adaptive noise cancelling–based methods showed promise. Jafarifarmand et al.46 investigated real-time processing and ocular artefact removal using ICA and adaptive noise cancelling, focusing on a small number of EEG channels, making it suitable for BCI applications.
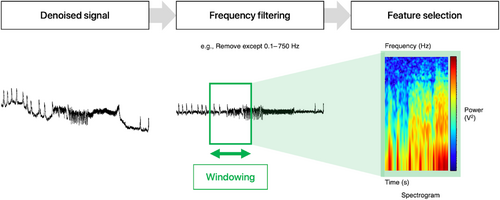
4.3 Feature extraction
Broadly, BCI signal processing, following denoising, involves feature extraction and classification (Figure 3). Task-specific features are extracted from EEG/ECoG signals using various methods. Feature extraction, in essence, involves frequency filtering, windowing, feature extraction, and feature selection which outputs selected features for the classifier. In BCIs, band power features represent the power of signals (relative amplitude) for a frequency band (a certain range of frequencies) averaged over a time window. Common feature extraction techniques are fast Fourier transform, autoregressive model, WT (shared between denoising and extraction techniques), and common spatial pattern. Although a brief comparison of the techniques is shown in Table 2, review articles with a specific technical focus on such techniques may provide the reader with an enhanced level of understanding.47, 48
| Technique | Advantages | Limitations |
|---|---|---|
| Fast Fourier transform | Rapid | Lacking in spatial resolution |
| Accurate at extracting frequency composition | Substantial trade-off between time resolution and frequency resolution | |
| Autoregressive model | Good frequency resolution | Validity depends on the proper selection of the model subtype |
| Improved frequency resolution for short time windows over fast Fourier transform | ||
| Wavelet transform | Considers different window sizes for different frequencies | Validity depends on the proper selection of the appropriate mother wavelet |
| Reduced trade-off between time resolution and frequency resolution | ||
| Improved performance in high and unstable frequencies | ||
| Common spatial pattern | Well-suited for multichannel signal analysis | Effectiveness depends on the individual-specific frequency band |
The main consideration underlying various techniques is the forced trade-off between time resolution and frequency resolution. The trade-off exists because when the time window for analysis is fixed, the longer the length of the window used, the higher the frequency resolution and the lower the achieved time resolution. This trade-off is named the uncertainty principle of signal analysis, analogous to Heisenberg's Uncertainty Principle commonly applied in quantum mechanics.49
4.4 Classification
Once features are extracted and selected, classification can be conducted to predict target variables from those discriminative features. Linear discriminative analysis is a popular classifier in EEG-based BCI applications due to its low computational requirements and has been successfully used for classifying right- and left-hand MI. However, it performs poorly on complex non-linear EEG data.50 Support vector machine is widely used in BCI research, as it is known for its good generalisation properties across individuals and performance with high-dimensional data. Meanwhile, neural networks offer a reasonable trade-off between accuracy and speed, making them extensively used in BCI research. Deep learning further improves accuracy in classification, by overcoming the obstacle of arbitrarily selecting weights within traditional neural networks. A neural network is a series of nodes. Within each node is a set of inputs and corresponding weights. Weights convey the importance of that corresponding feature in predicting the final output (classification), and deep learning attempts to optimise the weights themselves. Recent applications of deep learning include the classification of multiclass MI tasks.51
5 EFFECTORS AND APPLICATIONS
5.1 Directions for BCI applications
The current BCI research revolves around two arms, propelled by both publicly and privately sponsored research efforts. The Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative of the National Institutes of Health (NIH) has funded hundreds of academic, neuroscientific, and neuroprosthetic research programs, yielding major advances in neural interface technologies. Numerous companies (Emotiv Inc., Neuralink Corp., Kernel, Paradromics Inc., InBrain Neuroelectronics) and corporate ventures (Google Brain, Facebook Reality Labs) have also been established seeking to enable volitional interaction with prostheses and computers beyond pure clinical applications.52
5.2 Assistive communication and substitution
The first arm of BCI applications revolves around assistive communication or achieving the substitution of sensory and motor functions, and the second arm relates to closed-loop rehabilitation or neuromodulation. In assistive communication, brain activity is processed and used to generate a feed-forward route to control external devices. The prosthesis, the final stage of the BCI, can take many forms from motor to visual. A surrogate arm and hand may directly substitute biological functional deficits for patients with amputated arms, while a tool, such as a computer or a cursor, provides special functionality to the patient.
The identification of strong, reproducible brain signals can be used as the basis for driving a BCI, and foundational studies have demonstrated the ability to control various robotic arms with decoder outputs.8, 53 Motor neuroprostheses in humans have progressed dramatically in the past decades, with the milestones briefly summarised in Table 3. The field has developed from slow predictions to systems achieving the substitution of all limbs of the body.
| Year | Event | References |
|---|---|---|
| 1982 | Neurons in the motor cortex of non-human primates were found to be tuned towards the direction of movement | Georgopoulos et al., 198254 |
| 2000 | Cortical signals in non-human primates were successfully used for real-time control of robotic devices | Wessberg et al., 200055 |
| 2006 | First human implanted with a Utah array operates a BCI | Hochberg et al., 200656 |
| 2012 | Human achieves BCI-assisted self-feeding | Hochberg et al., 201257 |
| 2015 | BCI used to control a computer cursor | Aflalo et al., 201558 |
| 2015 | BCI used to virtually type on a computer | Jarosiewicz et al., 201559 |
| 2017 | BCI-based functional electrical stimulation used to restore whole-arm function | Ajiboye et al., 201760 |
| 2019 | BCI used to control a four-limb exoskeleton | Benabid et al., 201961 |
aBCI, brain–computer interface.
5.3 Motor imagery
A primary modality of assistive motor devices is based on MI, which involves imagining the execution of a movement, accessing motor representation consciously.62, 63 This can be done from a first-person (internal) or third-person (external) perspective. Research has shown that MI and motor representation share similar functional relationships and mechanisms, activating the same brain areas during actual and imagined movement.64, 65 Analysing EEG signals from the primary sensorimotor cortex during executed or imagined movement reveals activation of certain frequencies.66, 67 The variations in amplitude of such frequency bands, called event-related desynchronisation and synchronisation, correspond to specific states of movement.68 MI training improves both motor execution and neuroplasticity.62 However, MI abilities are subjective, requiring extensive assessment and training before application.69, 70
Hochberg's team53 first applied such findings to an individual with tetraplegia with a 96-channel MEA implanted in the primary motor cortex's hand area. Using algorithms to decode 2D movement intentions, the patient was able to open and close a prosthetic hand, control a computer cursor, and operate software and discrete neural states. This technology was further expanded to control robotic arms with up to three degrees of freedom (DOF), allowing hand grasping and self-feeding functionality.8 Other groups have devised similarly successful motor prostheses based on MEAs in the motor cortex, including success up to seven DOF.71 In 2019, Benabid and colleagues72 provided a proof-of-concept demonstration of a four-limb exoskeleton controlled by a fully implantable ECoG recording system with 64 epidural electrodes placed bilaterally over the motor cortices. The patient with tetraplegia achieved up to eight DOF without any recalibration over 7 weeks.
5.4 Functional electrical stimulation
Functional electrical stimulation (FES) is a technology that can reanimate paralysed muscles by delivering electrical stimulation to a muscle or the nerve innervating a muscle. Coordinated electrical stimulation can return function to any DOF of the body via synchronised muscle groups. FES can be deployed as the output of a BCI system to reanimate the upper extremity, using pattern recognition from residual functional muscles.36, 73, 74 Recent work has progressed further into individual reanimation of digits with a BMI-controlled FES system, using MI of the finger movements as a direct control signal for stimulation.75, 76
5.5 Communication prostheses
While most BCIs have primarily focused on motor replacement, recent advancements have expanded into interfaces for speech and communication restoration, which would be transformative for people who are unable to communicate because of neurological impairments.77-80 Early attempts of EEG spellers revolved around expanding simpler MI of arm and hand movements to create interfaces for speech and communication replacement, achieving 1 to 5 characters per minute.81, 82 Subsequent iterations utilising visually evoked potentials to guide cursors or keyboard inputs have achieved speeds of 60 characters per minute, but the need for constant gaze direction and panels of flashing lights on a screen remain key obstacles to naturalistic real-life applications.83 Henderson, Shenoy, Hochberg, and colleagues80 subsequently demonstrated that high-performance assistive communication systems were possible through higher-density intracortical 96-channel silicon MEAs (Utah arrays) implanted in the hand area of the motor cortex, and even faster communication was achievable by decoding rapid sequences of highly dexterous MI, such as handwriting.79 The key criteria for clinical applications were now met, including communication being entirely self-paced with the eyes free to move, and sufficient accuracy offline with a large-vocabulary language model. While these strategies achieve speeds comparable to able-bodied smartphone typing speeds (12–19 words per minute),84, 85 this remains inferior to the average of 150 words per minute of natural speech.86
Going forward, natural speech–based approaches that directly translate neural activity into speech appear to hold the key to restoring intuitive communication. Initial strategies for neural decoding of speech production focused on the direct classification of speech segments such as phonemes or words.87 However, these attempts were limited to small vocabulary sizes and slow communication rates. By contrast, in addition to the capability to communicate unconstrained vocabularies at a natural speaking rate, direct speech synthesis can capture prosodic elements of speech that are not available with text output, such as pitch intonation.88 In 2019, Edward Chang and colleagues10 designed a neural decoder that leverages representations of vocal tract articulatory movements encoded in the speech motor cortex to synthesise high-fidelity intelligible speech. This was subsequently translated to a paralysed patient with anarthria, who used attempted speech to control algorithms that decoded intended words and sentences in real time. Decoding neural-activity patterns in the ventral sensorimotor cortex combined with a natural-language model providing next-word probabilities given the preceding words in a sequence enabled higher communication rates and accuracy.89 A follow-up study demonstrated the continued stability of the relevant cortical activity beyond 2 years following device implantation, and that silently attempted speech could be as effective as an alternative behavioural strategy to imagined speech and overt-speech attempts.90 Recent studies have further advanced the field, with decoding algorithms achieving higher communication rates at 62 to 78 words per minute,91, 92 and a bilingual speech neuroprosthesis capable of decoding cortical activity into sentences in both English and Spanish.93 The existence of shared cortical articulatory representations across languages may enable decoding multiple languages without the need to train language-specific models. This study also demonstrated stable decodable cortical activity 4 years after implantation. However, challenges remain in generalising these technologies to patients fully locked in with negligible residual vocal tract function, beyond the spectrum of imagined speech, silently attempted speech, and overt-speech attempts currently studied in patients with some residual vocal cord function. Imagined speech involves internally simulating speech without actual articulatory movements, which may engage different neural networks compared with overt or attempted speech. Given the diversity of pathologies that result in dysarthria, speech-decoding studies may also consider recording from intact speech production regions such as the posterior superior temporal gyrus (STG), supramarginal gyrus (SMG) afferent signal, or preserved aspects of the precentral gyrus to accommodate cortical forms of dysarthria.94
5.6 Closed-loop rehabilitation and neuromodulation
The other arm in BCI applications is closed-loop rehabilitation or neuromodulation, where the dynamic feedback loop aims to restore neural plasticity through reinforcement training or the modulation of brain processes. These closed-loop systems which respond dynamically to neurophysiological changes can also be termed adaptive neuromodulation.
A leading application of adaptive neuromodulation is in aDBS, also known as closed-loop DBS, which delivers stimulation in response to a specific electrophysiological brain marker that represents periods of aberrant neural activity in which stimulation would be needed, such as resolving freezing of gait in PD.95-98 Although conventional DBS has been clinically validated to reduce motor fluctuations in medically refractory advanced PD, many patients require a combination of levodopa and stimulation for best function. Constant stimulation disregards peaks and valleys in the effective brain concentration of levodopa, so patients may experience residual motor fluctuations that come with high or low dopaminergic states. aDBS could allow a reduction in stimulation current without loss of therapeutic benefit, reducing stimulation-induced adverse effects.
Traditionally, subthalamic beta oscillations (13–30 Hz) have been used as control signals due to their association with motor symptoms in PD. In proof-of-principle studies, Simon Little and colleagues99 demonstrated not only the feasibility of successful BCI-controlled DBS in patients with PD, but also the associated improvement in motor scores and reduction in necessary stimulation amplitude, which has an impact on both side effects and the lifetime of implanted battery systems. Alberto Priori's group100 subsequently studied aDBS effectiveness across 8 hours, demonstrating the potential for hours of optimised unrestricted patient activity, and in conjunction with levodopa. These initial studies recorded subthalamic oscillations via externalised leads and used external customised neurostimulators, until Helen Bronte-Stewart and colleagues97 demonstrated the tolerability and efficacy of aDBS using a fully implanted neurostimulator capable of concurrent neural sensing and stimulation. The common approach involved the use of beta oscillations as a control signal; however, during active stimulation, beta oscillations may become less reliable due to stimulation-induced suppression of beta activity.101-103 In a blinded, randomised, cross-over feasibility trial, Starr and colleagues11 demonstrated the efficacy of aDBS using stimulation-entrained gamma oscillations as control signals in patients with PD. The study found that stimulation-entrained gamma oscillations, centred at half the stimulation frequency (~65 Hz), in either the subthalamic nucleus or the motor cortex, served as optimal biomarkers of motor function fluctuations during ongoing stimulation. These gamma oscillations were robust across varying stimulation amplitudes and correlated with patients’ dopaminergic states, outperforming traditional beta-band signals in predicting motor symptoms. Multi-site brain recordings, including ECoG from the motor cortex, provided additional neural signals that were not detectable from subcortical recordings alone. By utilising these signals, the aDBS system could adjust stimulation parameters in real-time, improving motor symptoms and quality of life compared with clinically optimised standard stimulation. In some patients, cortical stimulation-entrained gamma activity was the only reliable biomarker for adaptive control, underscoring the added value of cortical recordings in aDBS, and may signal surgical implications for future iterations.
The potential to tailor stimulation parameters based on real-time neural feedback holds promise for enhancing therapeutic outcomes while minimising adverse effects in patients with PD and other neurological disorders. Similar aDBS modalities have been investigated in other conditions with varying levels of success, including essential tremor,104 obsessive-compulsive disorder,105, 106 and epilepsy.107, 108
5.7 BCIs as neurofeedback
In rehabilitation, traditional therapies for restoring motor function focus on the forced use of the impaired limb, for improving muscle strength and performance of activities of daily living.109, 110 However, the success of rehabilitation relies on the patient having some residual function. Alternative methods for restoring function have thus been studied, using BCIs as a neurofeedback tool to engage neuroplasticity along the corticospinal pathway. Users receive real-time feedback on particular neural activity features, such as oscillatory signals from the cortex, and learn to control the target neural signal. Literature has shown that functional recovery is often associated with cortical activity returning to a state close to that of an unimpaired individual.111, 112 Neurofeedback-driven BCI training can directly impact cortical activity and possibly restore regular cortical activation patterns, potentially benefiting downstream neuromuscular systems.
Recent studies have investigated the feasibility of restoring movement using BCI training in patients with chronic stroke. In early studies, patients learned to modulate their brain activity in order to control an orthosis that opens and closes the hand, which led to improvements in motor function.113-116 In one study, participants with chronic hand weakness secondary to stroke underwent training to modulate sensorimotor rhythm amplitudes activated during imagery of movement of the paralysed hand, as measured by an MEG-based BCI system.117 Drawing inspiration from Hebb's rule for neuroplasticity where ‘the neurons that fire together, wire together’,118 the sensorimotor rhythm amplitude was set to control the vertical position of a computer cursor. In addition, the same neural signals were simultaneously used to control the movement of a hand orthosis. Remarkably, most patients were able to achieve control with MEG sensors at sensorimotor areas ipsilateral to their subcortical stroke lesions, suggesting that imagery training can lead to cortical reorganisation and enhanced neurocognitive rehabilitation when coupled with somatosensory feedback.
Another feasibility study tested an EEG-based BCI system that used signals related to affected hand MI, recorded from the unaffected hemisphere, to control the affected hand via a powered exoskeleton.119 The unaffected hemisphere demonstrated strong potential in the BCI rehabilitation system which improved patients’ Action Research Arm Test (ARAT) scores surpassing the minimal clinically important difference.120 In a transcranial magnetic stimulation–based study, neurofeedback increased the corticospinal excitability of affected muscles in patients with stroke with severe upper limb paralysis.121 Taken together, these results build on previous evidence that BCI-controlled rehabilitation systems can facilitate motor recovery.122
5.8 Prospective rehabilitation in neuropsychiatry
Closed-loop BCIs may have further applications in neuropsychiatric illnesses. It has been shown that sensory-affective experiences can be modulated by a BCI in real time in freely behaving rats.123 By coupling neural codes for nociception directly with optogenetic activation of the prelimbic prefrontal cortex, the BCI effectively inhibited affective behaviours caused by a multitude of pain, including acute mechanical or thermal pain, and chronic inflammatory or neuropathic pain. Furthermore, modelling frameworks have recently been developed to decode mood state variations from intracranial recordings in patients with epilepsy.124, 125 Similar to how motor BCIs encourage patients to learn to better control them through neural adaptation, driven by somatosensory feedback of the decoded output, providing explicit feedback of the decoded mood state to the patient may lead to successful rehabilitation where patients learn to control their own mood states.126, 127
6 THE HONG KONG EXPERIENCE
Significant contributions towards the BCI field have been made by local researchers, with various research laboratories focusing on practical robotics for post-stroke rehabilitation, or signal decoders facilitated by state-of-the-art algorithms. The practical local experience has largely focused on broader human–machine interface technologies which may or may not involve cortical input.
Considerable progress was made before the turn of the millennium, demonstrating the practicality of gait analysis using gyroscopes128 and, by extension, wearable sensing and feedback systems, by Professor Raymond Kai-Yu Tong, who would later lead research teams at multiple local institutions. Subsequently, multiple generations of non-invasive exoskeletal hand robotic training devices have been developed, for patients with stroke to retrain motor functions of their impaired hand. The system functions with EMG signals from the hemiplegic hand muscles as the afferent signal, therefore detecting the movement intention, and the exoskeleton effector assists the patient in limb movements.129, 130 The goal would be limb rehabilitation leading to significant functional improvements, rather than motor substitution with complete dependence on the system. Crucially, these interactive treatments were more effective in improving muscle coordination and reducing spasticity, compared with passive robotic-powered treatments without patient control through EMG.131
Local research teams have also contributed towards the development of BCI-based neurorehabilitation approaches through advanced EEG feature expression technologies. BCIs based on MI have overwhelmingly focused on supporting the rehabilitation of motor functions of upper rather than lower limbs, due to the relative difficulty of detecting lower limb MI represented deeper within the sensorimotor cortex. Professor Choi's group132 has proposed innovative techniques to reliably detect MI of lower limbs, through a new paradigm of walking imagery in a virtual environment, supported by a state-of-the-art multi-view multi-level deep polynomial network. The group has proposed other paradigms that attempt to address the most pertinent limitations in BCI input feature extraction, including a novel knowledge-leverage–based support matrix machine which reduces the amount of labelled EEG data needed from the target BCI subject in model training, thus potentially shortening the calibration process.133 Along the lines of improving generalisability and reducing costs of MI training, novel pipelines including a few-shot learning method, which encodes temporal patterns between MI trials, and a shallow Inception Domain Adaptation framework to extract features from data of multiple subjects have also been recently published by the group.134, 135 Resource-minimisation studies have also been conducted, where a minimal set of electrodes could be used for an individual with stroke for MI, to maintain 90% accuracy in BCI training sessions.136
7 FUTURE DIRECTIONS
Existing BCI technologies, if implanted in a wireless, compact form factor, could theoretically restore multiple DOF of movement in selected patient groups.8, 74, 137 Technological advancements in classification algorithms and novel electrodes involving deep learning may further enable order-of-magnitude improvements in BCI performance.
7.1 Advancements in BCI electrodes
The Utah array has been widely used in human BCIs with 48 implants as of 2018.138, 139 While no replacement designs come close to demonstrating similar robustness and clinical dependability over a decade, pre-clinical testing has revealed promising electrode designs that could soon enter clinical use. Devices such as Neuropixels with 384 channels and other high-density silicon probes allow far greater channel count in rodent experiments.16, 140, 141 Invasive intracranial electrodes currently used in BCIs are metal based, which presents significant distortion when capturing the full spectrum of electrophysiological signals, where low frequencies tend to be attenuated and distorted.142 Field-effect transistors have been developed as a non-inferior alternative. As they are active devices, they can act as inherent signal amplifiers to increase the signal-to-noise ratio without interference from pre-amplified noise from electrical connections.143 Novel materials promise to improve electrochemical inertness, electrical conductivity, and biocompatibility to produce flexible and ultrathin devices. These include, among others, graphene,18 carbon fibres,144 and silicon carbide.145 These electrode materials may allow designs smaller than neurons promise to drastically reduce bleeding and gliosis, opening further possibilities for high-channel-count chronic BCI implants.146
7.2 Challenges in BCI adoption
In April 2021, the FDA authorised the first brain-controlled hand exoskeleton for neurorehabilitation, IpsiHand by Neurolutions. This device represents a step towards integrating BCIs into rehabilitative care. However, several challenges must be addressed before wider adoption can occur, such as patient stratification and personalised treatment, integration into existing treatment plans, and commercialisation of BCIs.
Patients selected for BCIs, including survivors of stroke and spinal cord injury, exhibit a high degree of heterogeneity, requiring personalised rehabilitation plans.147 Factors such as BCI training duration and intensity should be tailored to individual patients—yet evidence is currently limited as to which patients benefit most from BCI-enabled neurorehabilitation training given publication bias towards successful cases, and more research is needed to establish optimal training parameters. Clear definitions of inclusion and exclusion criteria are a crucial foundation for comparisons across BCI frameworks, and to determine a significant level of efficacy.
Integrating BCIs into existing treatment plans remains a key challenge, as rehabilitation approaches vary across different centres. Implants for neurological conditions are also frequently viewed as last-resort options, limiting sample size. Early adopters, who are open to innovation, are crucial in promoting BCI use among clinicians and patients as they may help shape future workflows.148 Similar to biomedical devices such as open-loop deep brain stimulators, the commercialisation of advanced BCIs faces several obstacles, including complex certification processes, which vary geographically and have limited distribution pathways. Reimbursability is also a critical factor, as health insurances require clear, evidence-based benefits before covering costs for medical devices or treatments.
Technical hurdles must be overcome to demonstrate clinical efficacy and safety in sufficiently large cohorts. Obtaining safety and efficacy data is a long and costly process, and the commercialisation of academic work is hampered by long regulatory processes and technology risks. Furthermore, academic funding, degree cycles, and publishing dynamics rarely offer support to these multiyear studies with uncertain success. Despite challenges, recent studies have successfully demonstrated the clinical efficacy of select implantable neural interfaces, highlighting the need for sustained public funding and partnerships between academia and industry to advance the translation of BCIs. Scalable manufacturing design, quality control comprehension, and engineer-friendly system designs are crucial investments to further BCIs beyond proof-of-concept studies.149-151
8 THE ETHICS OF BCIs
The emergence of neuroethics as a distinct subfield of bioethics reflects the growing need to address ethical considerations specific to neurotechnology and brain research.152 The term was formally established at the 2002 Dana Foundation conference ‘Neuroethics: Mapping the Field’, where it was defined as ‘the examination of what is right and wrong, good and bad about the treatment of, perfection of, or unwelcome invasion of and worrisome manipulation of the human brain’.153 While neuroethics encompasses both the neuroscience of ethics and the ethics of neuroscience, this discussion focuses on the latter in the context of BCIs.
Drew154 published the article ‘The ethics of brain–computer interfaces’ in Nature which provides a good reference on the history of ethical issues of the interaction between human brains and computers. DBS was first approved by the FDA in 1997 for idiopathic PD. The subsequent expansion of DBS applications to conditions such as obsessive-compulsive disorder and depression has raised fundamental questions about personhood and identity. The observation that subthalamic nucleus stimulation may induce impulse control disorders illustrates the complexity of these interventions. Although alternative targets such as the globus pallidus interna may circumvent specific complications, the broader philosophical question persists: to what extent can we modify neural function while preserving individual identity?
A systematic review by Burwell et al155 highlighted ‘humanity and personhood’ as a central ethical concern in BCI implementation. One could debate if a BCI implant would become a part of the patient's body schema—that is, the distinction between augmentation and incorporation. While the spectre of ‘cyborgisation’ often dominates public discourse, it is crucial to recognise that chronic illness itself can profoundly alter personal identity. Moreover, in conditions such as locked-in syndrome, BCIs may actually restore rather than compromise personhood by enabling communication and environmental interaction.
The concept of autonomy presents particular challenges in the BCI context. The engineering perspective emphasises functional autonomy—the restoration of bodily control and environmental interaction. However, the neuroethical framework considers autonomy as the capacity for intentional action independent of external influence.156 This raises complex questions about agency and responsibility: how should we attribute causation when BCIs interpret and act upon suppressed neural signals? Would patients be able to attribute any unethical or illegal acts to interference by their BCI implants? Could we disregard all potential involvement of a BCI implant? The legal and autonomy implications of these questions warrant careful consideration as BCI technology advances.
Research ethics and informed consent require particular attention in BCI implementation. The consent process for invasive BCIs must address not only therapeutic considerations but also the potential uses of acquired neural data. Patients must be empowered to make informed decisions about the use of their brain signals for research, model development, and commercial applications. Data privacy and security considerations take on heightened significance in BCI applications, as neural signals constitute highly personal information.157 The storage, transmission, and processing of these data require robust safeguards to protect patient confidentiality while enabling necessary clinical and research applications. This balance becomes increasingly critical as BCI technologies advance and generate increasingly detailed neural information.157 Furthermore, clinicians have an obligation to present a comprehensive discussion of alternatives, including non-invasive assistive technologies and conservative management approaches. Besides, the experimental nature of BCI should be clearly acknowledged, given the current limitations in evidence for therapeutic efficacy.158
As BCI technologies continue to advance, the field must develop robust ethical frameworks that evolve alongside technological capabilities. These frameworks should not only address current challenges but also anticipate future ethical considerations as the technology becomes more sophisticated and pervasive in clinical practice. Success in this endeavour will require sustained collaboration between clinicians, researchers, ethicists, and regulatory bodies—a partnership that must balance rigorous patient protection with the pioneering scientific pursuit that has characterised this revolutionary field since its inception. Only through this careful balance can we ensure that BCI development continues to expand its potential while upholding the highest standards of clinical and ethical practice.
9 CONCLUSION
Our growing knowledge in BCIs ushers in an exciting new age of novel tools in assistive substitution and closed-loop rehabilitation. Although the field has yet to mature, encouraging efforts from Hong Kong scientists and clinicians have illustrated how BCI could be incorporated into our local healthcare. Future research should utilise digital-age infrastructure available at the Hospital Authority IT Innovation Office and the public–private collaborations such as the Science Park. BCIs remain at the forefront of the intersection among clinical neurosurgery, computer science, and electrical engineering, and promise to revolutionise neurological care for our patients.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




