Potential postmortem microbial biomarkers of infant and younger children death investigation
Abstract
Microbial communities associated with the human body are highly dynamic and reflect the host environment and lifestyle over time. Studies show death is no exception, with data demonstrating similar antemortem and postmortem microbiomes up to 48 h following death. These predictable microbial biomarkers can inform death investigation by helping to estimate the postmortem interval and build models to identify cause and manner of death. However, no attempts have been made to model potential microbial biomarkers in pediatric (≤2 years) deaths. This study provided a cross-sectional survey of the microbiota of 53 pediatric cases (black, white, both sexes) seen in Wayne County, Michigan. Autopsy cases represented accidents, homicides, or natural causes. Postmortem microbiome were collected by swabbing the eyes, ears, nose, mouth, umbilicus, brain, rectum, trabecular space, and cardiac blood. 16S rRNA sequence analyses indicated that sex, race, age, body site, and manner of death (MOD) had significant effects on microbiome composition, with significant interactions among MOD, race, and age. Amplicon sequence variants identified intra- and interhost dispersion of the postmortem microbiome depending on death circumstance. Among manners of death, non-accidental deaths were significantly distinct from all other deaths, and among body sites the rectum was distinct in its microbial composition. There is a real need for robust postmortem microbiome before it can be standardized as a practical tool for use in forensic investigation or public health. These results inform postmortem microbial variability during pediatric death investigation that contributes to a larger effort to understand the postmortem microbiome.
Highlights
- First survey of pediatric (≤2 years) postmortem microbiome using high-throughput amplicon sequencing.
- Sex, race, age, body site, and manner of death had significant effects on microbiome structure.
- Pediatric postmortem microbiota could be a potential tool to assess health preceding death.
- This study is foundational for future analysis of microbiomes in routine death investigation.
1 INTRODUCTION
While the importance of microbiota to human health has been a subject of speculation since the early 1900s, the potential of the human microbiome as a tool to provide insight into the human health condition became a subject of interest within the last decade [1-3]. With advancements in DNA sequencing and bioinformatic technologies, studies have demonstrated that from birth, microbes are a substantial presence on and within the human body, thought to harbor an estimated 500–1000 bacterial species and up to 20 million microbial genes [4, 5]. This microbial diversity far exceeds the genetic diversity of the human genome, and also varies by anatomical site, with distinct microbial communities observed in regions such as the eyes, ears, nose, mouth, gut, rectum, hair, and skin [4, 6, 7]. These microbial communities are specific to an individual, not unlike a fingerprint, but are also highly dynamic and variable through human development and aging. They adapt to the host environment, physiology, and lifestyle over time [8-10]. Studies have shown this pattern continues even after death, with postmortem microbiomes resembling antemortem microbiota up to 48 h postmortem [7], and in some cases as late as 72 h [11, 12]. In this way, human postmortem microbiota have a unique potential to inform our understanding of both human health and death investigation. However, studies of the human microbiome are predominantly antemortem, have been primarily limited to adults, and have rarely examined pediatric deaths.
Like the antemortem microbiome, bacterial communities differ among body sites such as the skin, mouth, and gut, and change in a predictable fashion during decomposition, with certain taxa associated with different taphonomic stages [11, 13-15]. Investigations of postmortem human microbiota vary in focus and extent; some examine a single body site whereas others address multiple sites in multiple cases. Nearly all results are limited by the availability of human data representative of real-world community demographics. Additionally, there are fewer data available for postmortem microbiota of children and infants, with the exception of a study from Pechal et al. [16] examining the frozen and thawed remains of two children during autopsy through 16S rRNA sequencing, and several large-scale reviews and surveys by Pryce et al. [17, 18] and Weber et al. [19] evaluating culture-based bacterial findings from postmortem sampling of sudden unexplained death in infants (SUDI) in the United Kingdom. As evidenced by significant inter-person antemortem microbiome variation, more robust postmortem microbiota data are needed across age ranges and demographics in order to provide a more cohesive understanding of these communities after death in infants and younger children [6, 8, 20-22].
Infant mortality rates in the United States exceeded a total of 19,000 deaths from 2007 to 2020, or 6 per 1000 live births (Table S1). This rate in Wayne County, Michigan is just over 1.5 times that of the United States [23] (Table 1). A prevalent cause of death in infants is sudden unexpected infant death, or sudden unexpected death in infancy (SUID), which is now recognized as a broad category that encompasses cases attributed to any sudden or unexpected death of an infant with known or unknown causes [24, 25]. Falling into this category is sudden infant death syndrome (SIDS), a term used to describe the sudden, unexplained death of otherwise healthy infant while sleeping [24, 26]. Another significant cause of death falling under SUID is also co-sleeping, wherein an infant sleeps with a parent, sibling, or other caretaker in an adult bed, on a couch, or any area other than an infant crib by themselves [27]. Studies have shown that this can create an unsafe sleeping situation for infants, as they are obligate nasal breathers and their airways may be easily blocked by the body of another person, a cushioned surface, or blankets, leading to asphyxiation [28-30]. Complications can arise when an unexplained infant death occurs, as SIDS and co-sleeping deaths present very similarly, and autopsy findings can rarely distinguish between them [27, 28, 30]. Addressing this problem of determining manner of death in suspected SIDS cases, framed within the context of the postmortem microbiome, was the aim of this study.
| Sample size (cases) | Age range | Median age | Race | Sex | Years collected | Body sites collected |
|---|---|---|---|---|---|---|
| 53 | 0–817 Days (0–27 months or 0–2.22 years) | 109 days (3.6 months or 0.3 years) | 40 Black; 13 White | 26 males; 27 females | 2015–2022 | Ears, Eyes, Nose, Mouth, Umbilicus, Rectum, Trabecular Space, Interhemispheric Fissure, Cardiac Blood |
The potential of the postmortem microbiome to reveal information about antemortem health and manner of death was demonstrated in adults by Pechal et al. [7], who observed evidence for decreased microbial diversity in individuals with heart disease as well as indicator taxa related to cases resulting in violent deaths. Using machine learning algorithms, Zhang et al. [20] showed that bacteria from the eyes were the most informative anatomical site to predict manner of death, though rectal communities were highly accurate models, and that different combinations of body site microbes increased each algorithm's accuracy. These results show the potential of the postmortem microbiome as a predictive tool to not only survey the living health condition but to also inform forensic investigations, especially in cases of unexpected death. Investigative methods following sudden unexpected deaths in infancy vary internationally, and while some recommendations have been proposed there is currently no routine, standardized postmortem microbiology sampling procedure utilized during forensic autopsy for adults or infants [31, 32]. Further, surveys of pathologists from 16 countries across Europe and Turkey show that while most made use of postmortem microbiology during autopsy, the most frequently requested analyses by far (96%) were culture-based methods, with 16S rRNA sequencing being the least requested at 6% [31]. Methods and protocols described by Pechal et al. [8] to sample and sequence the postmortem microbiome could be integrated into routine autopsy procedure, and analyses for biomarkers may be developed into analytical workflow recommendations to aid in forensic investigation as proposed by Kaszubinski et al. [33]. Further analyses, standardization, and robust datasets are needed before tools such as this can be feasibly integrated into real world scenarios and accepted into a court of law [34, 35].
As the microbiome rapidly changes during the first 3 years of life, this could be a critical time to better understand microbial community dynamics in infants and young children [3]. To address postmortem microbiomes of infants and children, we conducted a cross-sectional survey of 53 pediatric autopsy cases using 16S rRNA sequencing with the goals of (1) characterizing the composition of the infant postmortem microbiome for the first time; and (2) identifying potential microbiome biomarkers that could predict manner of death. We hypothesized that: (1) microbiome community composition would be structured by body site and by manner of death; and (2) that microbiome communities of co-sleeping (control) death cases would be more similar in composition than other manners of death, as such unexpected deaths are due to asphyxia of otherwise healthy infants and younger children.
2 MATERIALS AND METHODS
2.1 Case demographic information
Microbiome samples were acquired from the Wayne County Medical Examiner's Office (MEO) located in Detroit, MI, USA, through routine death investigations carried out since 2014. Only samples from deaths under 2 years or younger were analyzed within this study (henceforth referred to as “infant”). The following data were compiled for each of the 53 cases sampled: sex, race, date of birth, estimated age (days, months, and years), time of death, and season. In addition, the following information was collected from autopsy reports: autopsy date, height (crown-heel length, crown-rump length, and foot length) and height percentile, weight and weight percentile, manner of death, cause of death, and state of decomposition at the time of autopsy, as well as any other pre-existing conditions or medical history. All causes, manners of death (MOD), and postmortem interval (PMI) estimates were determined by a board-certified forensic pathologist. Research involving human remains from deceased persons does not necessitate an Institutional Review Board (IRB) evaluation. These cases are governed by the same regulations that apply to autopsy samples and do not fall under the category of human subject research. No deliberate tissue collection or extraction occurred during the gathering of microbial samples. Microbiological sampling is a recognized method used for pathological diagnosis.
2.2 Manner of death classification
For the purposes of this study, the manner of death for each case was categorized as one of the following: natural, accidental, non-accidental, or control. Natural death was assigned to cases where the circumstances of death were attributed to natural, pre-determined causes, such as a pre-existing clinical diagnosis. Accidental death was assigned to cases involving chance circumstances such as accidental drownings, or airway obstruction unrelated to co-sleeping with an adult. In contrast, non-accidental death was assigned to cases involving intentional harm caused by blunt force injury, drug toxicity, or other non-accidental injury. Finally, “control” cases were those where circumstances of death were due to asphyxia, airway obstruction, or over-lay attributed to co-sleeping. Studies have shown that the act of co-sleeping with a sibling or adult caretaker can create an unsafe sleeping environment for infants, as their airways may be easily obstructed by the body of another person, cushioned surfaces, or blankets, or weight on the chest may obstruct respiratory effort, leading to asphyxiation [27, 28]. Co-sleeping deaths were chosen as a control in this study based on the assumption that infants in this category were healthy and free from elements such as disease or bodily trauma that would have otherwise contributed to death.
2.3 Sample collection
Individual microbial communities were collected by trained autopsy personnel within the Wayne County MEO from each decedent using sterile, DNA-free cotton-tipped applicators (Puritan) and stored in 100% ethanol in sterile 1.5 mL microcentrifuge tubes (VWR International), as described in Pechal et al. [8]. Nine anatomical locations were sampled from each decedent: the ears, eyes, nose, mouth, umbilicus, rectum, trabecular space (TS), interhemispheric fissure (IF), and cardiac blood (CB). Anatomical sites were sampled by manually swabbing a single applicator over the area for at least 30 s and breaking off the cotton tip into an individual microcentrifuge tube. Samples were taken from cases within 24–48 h of admission to the Wayne County MEO, transported in cooler storage, and placed immediately in −20°C storage to await further processing.
2.4 DNA isolation, quantification, and confirmation
DNA was extracted from cotton swabs using the QIAGEN DNeasy Blood and Tissue Kit. Swabs were first prepared for lysis on Parafilm sheets (Bemis Company, Inc.) by using sterilized dissecting forceps and scalpels (Thermo Fisher Scientific) with sterile, single-use carbon steel blades (Surgical Design Inc.) to carefully remove cotton from the wooden applicator. The cotton was then transferred back into its original microcentrifuge tube, and the applicator and Parafilm were discarded. To preserve the individual microbial communities, new Parafilm sheets and sterile instruments were used to prepare each sample. The manufacturer's protocol was then followed accordingly with the addition of 10 μL of 15 mg/mL lysozyme per sample to promote microbial cell lysis. Samples were also treated with 4 μL 100 mg/mL RNase A following incubation per the MSU Genomics Core Illumina sample sequencing requirements, and final DNA elution volume was modified from 200 to 50 μL. DNA was quantified using a Qubit 2.0 fluorometer (Thermo Fisher Scientific) and a 1× dsDNA High Sensitivity Assay (Thermo Fisher Scientific).
The microbial gene amplicon V4 region was amplified using conventional PCR in triplicate with Invitrogen Platinum Green Hot Start Master Mix (2×) (Thermo Fisher Scientific) and region-specific primers 515f/806r (5′GTGCCAGCMGCCGCGGTAA, 5′GGACTACHVGGGTWTCTAAT). With a total reaction volume of 25 μL, the PCR reaction mixture contained 24 μL mastermix and 1 μL template DNA per sample, as well as E. coli and PCR-grade nuclease-free water as positive and negative controls, respectively. Reactions were prepared in a biological safety cabinet and amplified according to previously described methods [36] using a Veriti 96-well thermal cycler (Thermo Fisher Scientific). Amplification of 16S rRNA was confirmed by gel electrophoresis in 3% agarose gels (VWR International). GeneRuler 1 kb ladder (Thermo Fisher Scientific), amplicons, and controls (5 μL of each) were loaded and run at 90 V for 40 min. Gels were then agitated in GelRed solution (Thermo Fisher Scientific) for 30 min on a 3D platform rotator, then imaged using the Axygen Gel Documentation System (Corning Incorporated) at UV 302.
2.5 16S rRNA gene amplicon sequencing
DNA sequencing and library construction for 298 individual microbial body site samples was performed at the Michigan State University Genomics Core facility (East Lansing, MI, USA). The V4 region of the 16S rRNA gene was amplified using dual indexed 515F/806R Illumina primers (5′GTGCCAGCMGCCGCGGTAA, 5′GGACTACHVGGGTWTCTAAT) following the protocol described in Kozich et al. [37]. PCR products were batch normalized using an Invitrogen SequalPrep DNA Normalization plate (Thermo Fisher Scientific), and products were pooled and cleaned up using a QIAquick Spin column (QIAGEN) and AMPure XP magnetic beads (Beckman Coulter, Inc.). Library pools were quality controlled and quantified using a combination of Qubit dsDNA HS, Agilent 4200 TapeStation HS DNA1000 (Agilent Technologies, Inc.), and Invitrogen Collibri Illumina Library Quantification qPCR assays. Amplicon pools were then loaded onto an Illumina MiSeq v2 standard flow cell and sequencing was carried out in a 2 × 250 bp paired-end format using a MiSeq v2 500 cycle reagent cartridge. Custom sequencing and index primers complementary to the 515f/806r oligomers were added, base calling was performed by Illumina Real Time Analysis (RTA) v1.18.54, and output of RTA was demultiplexed and converted to FastQ format with Illumina Bcl2fastq v2.20.0. The data for this study have been deposited in the European Nucleotide Archive (ENA; https://www.ebi.ac.uk/ena/browser/home) under accession number PRJEB78864.
2.6 Bioinformatics
QIIME2 (Quantitative Insights Into Microbial Ecology) (v2023.2) was used to filter and analyze raw 16S microbial sequencing data. Demultiplexed reads were assembled, quality-filtered, classified, aligned, and contingency filtered to remove singletons and reads corresponding with mitochondria or chloroplasts using DADA2, a Naïve Bayes classifier (V4, SILVA database v138-99), and default settings in QIIME2 [38, 39]. Relative abundances of microbiome communities at the phylum (>1%) and family level among body sites and manners of death were analyzed in Rstudio (v4.3.1) [40] using the phyloseq (v1.44.0) [41], qiime2R (v0.99.6) [42], and microbiome (v1.22.0) [43] packages.
2.7 Statistical analyses
Permutational Multivariate Analysis of Variance (PERMANOVA) and FDR (False Discovery Rate) corrected pairwise PERMANOVA tests using weighted UniFrac distances were conducted using the phyloseq and vegan (v2.6–4) packages to evaluate the effect of body site, manner of death, and additional covariates (age, sex, race) on overall microbiome community composition. Pairwise Wilcoxon rank-sum tests of observed and Shannon diversity were conducted using the phyloseq and stats packages [40, 41] to test alpha-diversity differences among body sites and manners of death. Covariate relationships and microbiome structure were visualized with principal coordinates analysis (PCoA) using ggplot (v3.4.3). Weighted UniFrac metrics were used for these ordinations because they account for phylogenetic distances and relative abundances among microbes [44].
To identify potential biomarkers most likely to explain differences among significantly distinct microbiota, covariates were tested using the linear discriminant analysis (LDA) effect size (LEfSe) in the microbiomeMarker package in (Rstudio) [45, 46]. LEfSe is an algorithm that uses a Kruskal–Wallis rank sum test and pairwise Wilcoxon test to discover high-dimensional biomarkers that can statistically differentiate between biological groups [46, 47]. A heatmap was constructed using the microbiomeutilities (v1.00.17) [48] and pheatmap (v 1.0.12) [49] packages to visualize correlations among the top 15 most abundant ASVs and covariates of interest. All statistical analyses were conducted in Rstudio and all p-values were considered significant with alpha ≤0.05. The scripts used for this project are available via GitHub (https://github.com/jenpechal/pmm-pediatric).
3 RESULTS
16S rRNA sequences from a total of 298 body site samples (eyes, ears, nose, mouth, umbilicus, rectum, trabecular space, interhemispheric fissure, and cardiac blood) from 53 cases of routine infant and younger children autopsies collected from 2015 to 2022 in Wayne County, Michigan (Table 1; Tables S2 and S3). Manner and causes of death were categorized based on individual evaluation of circumstances described in autopsy and case reports (Table 2). There were also six cases wherein manner of death could not be conclusively determined with pathological findings from routine autopsy, referred to hereafter as indeterminate deaths, as well as six cases for which cause or manner of death were not available (“ND”). Due to the limited availability of samples from cases of infant death and comprehensive published findings of postmortem microbiome profiles in this age range, these cases were still included in analyses to support a database of postmortem infant microbiota. Samples from cases with a manner of death classified as Indeterminate or ND were filtered from the dataset for the purpose of alpha-diversity analyses.
| Manner of death | Cause of death |
|---|---|
| Accidental | Airway obstruction, asphyxia, drug toxicity, drowning |
| Non-accidental | Abusive head trauma, asphyxia, blunt force trauma, cranio-cerebral injuries, drug toxicity, multiple blunt force injuries, multiple blunt trauma, multiple stab wounds, premature birth |
| Natural | Acute bronchitis, complications of Edwards syndrome, congenital heart disease, pneumonia, RSV bronchiolitis, prematurity complications, sepsis due to urinary tract infection, status asthmaticus |
| Control | Airway obstruction or asphyxia due to co-sleeping |
| Indeterminate | Anoxic encephalopathy, fentanyl toxicity, fentanyl toxicity with complications, sudden death, suffocation, unknown |
3.1 Microbiome community structure
Infant postmortem microbiome were represented by four primary phyla: Actinobacteria, Bacteroidota, Firmicutes, and Proteobacteria (Figure 1; Tables S4 and S6). Among all body sites the most dominant phylum was Firmicutes (34.3%–62%), excluding cardiac blood samples which were predominantly Bacteroidota (41%). Second most abundant was Proteobacteria (22.1%–36.2%) among the interhemispheric fissure, trabecular space, eyes, ears, nose, and mouth samples. Among samples from non-accidental, natural, and control deaths the most dominant phylum was Firmicutes (41%–64%), while Proteobacteria was most abundant in accidental (41%) and indeterminate cases (32%).
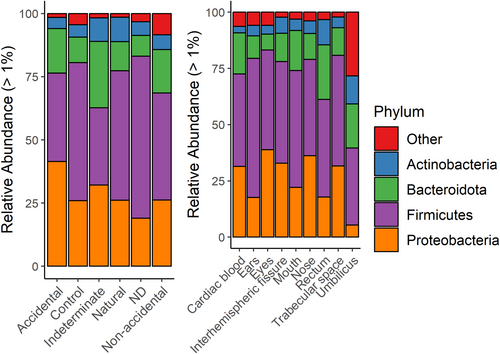
Relative abundances for the top 13 family-level taxa varied among body sites (Figure S1 and Table S5), with the most prevalent being Streptococcaceae (1.3% in rectum samples to 30% in mouth samples) and Enterobacteriaceae (2.5% in umbilicus samples to 19% in eye samples). Ear samples were represented by Staphylococcaceae (44.5%) more than any other site, while rectum samples had the highest abundance of Lachnospiraceae (12.7%) and Peptostreptococcales-Tissierellales (5.6%), but lacked Moraxellaceae, a family present at low relative abundance (0.6%–7.2%) in all other sites. Bifidobacteriaceae was also more abundant in the rectum (10.7%) and umbilicus (10.1%) than other body sites (<5%). Enterobacteriaceae was most abundant in accidental deaths (25%), and Streptococcaceae was present at 12%–26% relative abundance among all MODs (Table S7).
There were significant (p < 0.05) and highly significant (p < 0.01) differences in Shannon and observed amplicon sequence variants (ASV) alpha-diversity, respectively (Figures S3 and S4). For Shannon diversity showed there were significantly distinct bacteria communities between the cardiac blood, mouth, and nose, between the ears, nose, and mouth, and particularly between the rectum and all other body sites. The rectum had greater variability and mean alpha-diversity metrics among body sites. Tests of observed diversity showed significant differences particularly among the interhemispheric fissure, cardiac blood, rectum, and eyes, which appeared to be the most distinct compared to all other sites. While no significant differences were observed in ASV alpha-diversity measures between males and females, or black and white infants, significant differences were seen between cases aged 1 month or less, cases older than 5 months, and cases older than 1 year (Figure S5). Additionally, infant age appears to be positively correlated with both analyzed alpha-diversity metrics, which is particularly evident for Shannon diversity.
PERMANOVA results indicated significantly different beta diversity among body sites (F = 4.3441, p = 0.001) and MODs (F = 2.3776, p = 0.001) (Table 3), though the interaction of body site with MOD (F = 0.7443, p = 0.991) was not significant. Race (black or white) also had significant effects on beta diversity, as well as a significant interaction with MOD (F = 2.2626, p = 0.004). Sex (male or female) also had a significant interaction with MOD (F = 1.664, p = 0.020), but alone had no impact, while age significantly impacted beta diversity (F = 0.012, p = 0.012) and interacted significantly with sex (F = 1.664, p = 0.020). Principal coordinates analyses were plotted to visualize potential covariate interactions (Figure 2 and Figure S2). One interesting result was a distinct grouping of five samples from the same case of a non-accidental death (data near 0.0, −0.4) in each ordination. The decedent was a white male aged <1 day (the youngest case sampled) and the cause of death was determined to be asphyxia.
| Factor | Df | SS | R 2 | F | Pr (>F) |
|---|---|---|---|---|---|
| Body site | 8 | 3.438 | 0.124 | 4.34 | 0.001* |
| MOD | 5 | 1.176 | 0.042 | 2.38 | 0.001* |
| Age | 7 | 1.164 | 0.042 | 1.68 | 0.012* |
| Race | 1 | 0.253 | 0.009 | 2.55 | 0.011* |
| Sex | 1 | 0.071 | 0.003 | 0.721 | 0.669 |
| Body Site: MOD | 37 | 2.724 | 0.099 | 0.744 | 0.991 |
| Body Site: Age | 45 | 2.963 | 0.107 | 0.665 | 0.997 |
| MOD: Age | 14 | 1.867 | 0.068 | 1.35 | 0.039* |
| Body Site: Race | 7 | 0.432 | 0.016 | 0.624 | 0.983 |
| MOD: Race | 3 | 0.672 | 0.024 | 2.26 | 0.004* |
| Age: Race | 2 | 0.352 | 0.013 | 1.78 | 0.048* |
| Body Site: Sex | 7 | 0.426 | 0.015 | 0.616 | 0.974 |
| MOD: Sex | 4 | 0.694 | 0.025 | 1.75 | 0.018* |
| Age: Sex | 4 | 0.659 | 0.024 | 1.66 | 0.020* |
| Race: Sex | 1 | 0.025 | 0.001 | 0.251 | 0.988 |
| Body site: MOD: Age | 55 | 3.589 | 0.130 | 0.660 | 1.00 |
| Body site: MOD: Race | 8 | 0.601 | 0.022 | 0.759 | 0.848 |
| Body site: Age: Race | 4 | 0.180 | 0.007 | 0.454 | 0.994 |
| Body site: MOD: Sex | 18 | 1.066 | 0.039 | 0.599 | 1.00 |
| Body site: Age: Sex | 12 | 0.661 | 0.025 | 0.557 | 0.999 |
| Residual | 47 | 4.650 | 0.168 | ||
| Total | 290 | 27.662 | 1.00 |
- Note: Significant values (<0.05) are indicated with an asterisk (*). PERMANOVA test (weighted UniFrac distance) was conducted with 999 permutations.
- Abbreviations: DF, degrees of Freedom; SS, sum of squares.

Pairwise PERMANOVA was used to further identify significant differences among body sites and manners of death (Tables 4 and 5). Among manners of death, only non-accidental deaths were significantly distinct from all other deaths, and among body sites the rectum was distinct from all other sites excluding the umbilicus.
| Comparison (MOD) | p adj. | |
|---|---|---|
| Non-accidental | Natural | 0.294 |
| Accidental | 0.024* | |
| Control | 0.119 | |
| Natural | Accidental | 0.026* |
| Control | 0.257 | |
| Accidental | Control | 0.026* |
- Note: Values indicating significantly distinct microbiota (<0.05) are indicated with an asterisk (*).
| Comparison (body site) | p adj. | |
|---|---|---|
| Ears | Eyes | 0.058 |
| Nose | 0.029* | |
| Umbilicus | 0.207 | |
| Rectum | 0.005* | |
| Trebecular Space | 0.107 | |
| Mouth | 0.036* | |
| Interhemispheric Fissure | 0.089 | |
| Cardiac blood | 0.059 | |
| Eyes | Nose | 0.353 |
| Umbilicus | 0.107 | |
| Rectum | 0.005* | |
| Trebecular Space | 0.125 | |
| Mouth | 0.036* | |
| Interhemispheric Fissure | 0.107 | |
| Cardiac blood | 0.058 | |
| Nose | Umbilicus | 0.107 |
| Rectum | 0.005* | |
| Trebecular Space | 0.107 | |
| Mouth | 0.107 | |
| Interhemispheric Fissure | 0.099 | |
| Cardiac blood | 0.016* | |
| Umbilicus | Rectum | 0.078 |
| Trebecular Space | 0.107 | |
| Mouth | 0.099 | |
| Interhemispheric Fissure | 0.133 | |
| Cardiac blood | 0.141 | |
| Rectum | Trebecular Space | 0.005* |
| Mouth | 0.005* | |
| Interhemispheric Fissure | 0.005* | |
| Cardiac blood | 0.005* | |
| Trebec | Mouth | 0.059 |
| Interhemispher | 0.906 | |
| Cardiac blood | 0.323 | |
| Mouth | Interhemisphreric Fissure | 0.036* |
| Cardiac blood | 0.014* | |
| Interhemispheric Fissure | Cardiac blood | 0.650 |
- Note: Values indicating significantly distinct microbiota (<0.05) are indicated with an asterisk (*).
3.2 Identification of potential biomarkers
To identify potential biomarkers most likely to explain differences in microbiome structure representing covariates, linear discriminant analysis effect size (LEfSe) analyses were performed for all covariates, and significant results were visualized as a heatmap (Figure 3). There were only significant bacterial taxa representing sex and race microbiomes. Features most likely to explain differences based on race were Leptotrichiaceae (p = 0.0098), Sneathia (p = 0.006), and an uncultured Sneathia species (p = 0.001). These taxa were associated with white cases that appear to correlate most closely with a male and non-accidental death (Figure 3). However, a much lower prevalence of Sneathia is also seen in alignment with black, male, and non-accidental covariates. Results of LEFSe analysis based on sex resulted in one identified feature, Escherichia-Shigella (p = 0.010), which grouped microbiota associated with the female sex, and based on the heatmap also appear to correlate most distinctly with cases of white, accidental death.

4 DISCUSSION
Understanding the human microbiome has great potential to improve our understanding of the human health condition. Studies of microbiome structure and function can reveal a wealth of information about individual health and lifestyle, and as this evidence grows it informs the way diseases may be treated, diagnosed, and prevented [5]. With advances in microbiome science for antemortem health, the resilience and utility of human postmortem microbiota are frequently overlooked relative to studies of the living human microbiome. Even so, studies have established that postmortem microbiota play a significant role in decomposition, with temporal patterns of microbial succession and distinct microbial communities representing anatomical sites on human remains and other mammalian proxies [13, 15, 16], including manner of death [7, 33, 50]. With much to be learned from the postmortem microbiome before it can be standardized as a practical tool for use in forensic investigation or public health, there is a real need for robust, cross-sectional data representative of human microbiota of all ages and demographics. To address this need, this study presents the first survey of pediatric postmortem microbiome that contributes to a larger effort to understand the postmortem microbiome.
Investigation of the sudden unexpected death of an infant is challenging in many ways. For investigators it is critical to remain appropriate and highly sensitive to the nature of these deaths, while also conducting thorough examinations of the scene and circumstances of death, gathering relevant medical history and family testimony, and collecting time-sensitive forensic evidence [51]. Despite surveys showing pathologists' high opinion of its usefulness, complicating these investigations further is a lack of standardized sampling and analytical protocol for microbiological evidence [31, 32, 52]. A significant majority of microbiological sampling and analysis is also culture-based only, and it is clear that newer next generation sequencing tools like those used in this paper are not well known and are under-utilized [31]. Fernandez et al. [32] present detailed guidelines created by a multidisciplinary team of microbiologists, forensic pathologists, and forensic physicians to optimize and standardize microbiological sampling, including molecular techniques when necessary, and stresses the importance of bacterial evidence in determining a cause of death in cases of SUIDS. While some common practices appear to be used by pathologists, there is still significant room for standardization of microbiological evidence collection during routine autopsy, and an even greater need still within the field of forensic microbiome research.
While body site and manner of death individually had a significant effect on infant postmortem microbiome composition, there was no evidence to suggest yet that circumstances of death in infants are correlated with bacterial community structure in specific anatomical sites. Our results are consistent with other studies of body site variation in the antemortem human microbiome [6, 53], and our findings of the adult postmortem microbiome [7]. Of all covariates our results indicated significant interactions between manner of death and sex and race. This was not an anticipated result and contradicts findings by Pechal et al. [8] which demonstrated a lack of significance (p > 0.6) of race in structuring the postmortem microbiome in adults. Fortenberry [54] urges caution in examining racial and ethnic categories due to their lack of biological significance and reliability in determining human genomic diversity, while results from Huttenhower et al. [55] indicate a strong relationship between race/ethnicity and microbial community composition. Other studies have demonstrated consistent associations with ethnicity and bacterial abundance in body sites like the mouth, gut, and vagina, with the aim of understanding differential disease susceptibility and addressing health conditions such as bacterial vaginosis that disproportionately affect minorities [56-59]. Studies have also indicated that racial and ethnic status can affect rates of breastfeeding [60] which is thought to affect infant microbiome structure [61, 62], but we did not assess feeding strategies (e.g., breast vs. formula fed) in this research. There is some evidence of birth mode where minority women have higher rates of C-section [63, 64], but this relationship is not entirely certain [60-62, 65]. However, the role of birth mode in structuring the infant microbiome is unclear (e.g., Dos Santos et al. [66]). Nevertheless, additional research is needed to better understand factors that affect relationships of the antemortem microbiome and those found postmortem associated with different manners of death. Our results here are one of the first contributions to a better understanding of infant microbiomes with different manners of death.
Overlap of sex, race, and manner of death (Figure 3) implicated a white, male, non-accidental (homicide) case of infant death as the origin of the Sneathia marker. The cause and manner of death assigned by the MEO for this case were asphyxia and homicide. Sneathia is a genus belonging to Leptotrichiaceae, a family of obligate or facultatively anaerobic microorganisms [67]. Species of Sneathia are not only part of the normal flora of the mouth, gut, cervix, and vagina but also are associated with a number of clinical conditions including spontaneous preterm birth and labor, and preterm prelabor rupture of membranes (PPROM) [67]. Additionally, Sneathia is associated with bacterial vaginosis, may be capable of infecting the upper respiratory tract during pregnancy, and one species, Sneathia amnii is even documented invading fetal membranes and jeopardizing tissue viability [67]. Preterm birth has been declared the second leading cause of neonatal death worldwide, and Sneathia species detected in early-mid pregnancy may be associated with spontaneous preterm birth [57, 68]. Based on the information available about manner and cause of death (non-accidental, asphyxia) in the infant case clearly associated with this genus, it is unclear as to the mechanism of colonization leading to this potential biomarker becoming part of the infant microbiome, or how many and which body sites it is detected in. Nevertheless, our methodology has succeeded in measuring infant postmortem microbiomes significantly associated with antemortem traits and manners of death. These data are exciting and underscore the importance of continued research exploring and validating this and other potential biomarkers in a larger cohort of cases.
Age had a significant effect on infant microbiome composition, including a positive relationship of alpha-diversity with age (Figure S5). The development of the living infant microbiome has been studied at length, and this early life process is a critical window for proper immunological development, but not all sources and exact mechanisms of transmission are understood [69]. Infants most likely obtain microbes vertically and horizontally, receiving their first microbes from their mother via maternal seeding, and from the surrounding community post-birth [69]. This community or environmental effect has also been described by Pearson et al. [70] who observed the relationship between neighborhood environmental conditions and the human microbiome. Mallott et al. [58] also demonstrated increased infant microbes over time, with a significant shift observed at or just after 3 months of age associated with race and ethnicity. This is likely due to many factors such as varying diets (breastfeeding vs. starting solid food), association with green spaces, and social exposure from other children or caretakers [58]. While we did not observe a similar shift at 3 months, samples in the age range of 165–380 days old (5.5–12.7 months) had more significantly distinct pairwise differences in Shannon diversity.
Based on the significant effect of manner of death on postmortem microbiota presented here, additional surveys and in-depth analyses may uncover more specific biomarkers that more clearly indicate correlation with circumstances of death. These types of data have already been explored as such from the human postmortem microbiome, and when analyzed using machine learning algorithms showed increasing predictive power and accuracy with the incorporation of microbiota from more anatomical sites [29]. Pechal et al. [16] also used a similar approach, using bacterial communities and general additive models to estimate physiological time as a measure of carrion decomposition. These modeling strategies exhibit marked potential to improve accuracy rates in the classification of infant manner of death when traditional autopsy examination fails to ascertain why an infant death occurred due to a lack of bodily evidence or injury. Powerful tools that utilize machine learning strategies for biomarker discovery continue to multiply as interest in the human microbiome and potential biomarkers grows [71]. We recognize a limitation to the data presented are they encompass deaths from a single dataset collected from a defined geographic region (e.g., US Midwest) collected across several years. Postmortem microbiome data are not routinely collected in medical examiner's offices. Thus, it has not been established how the postmortem microbiome of infants and young children will differ when collected from additional geographic and/or socioeconomic groups.
5 CONCLUSION
This study presents a first comprehensive survey of the postmortem infant microbiome using 16S rRNA sequencing and provides support for differences in anatomical site and manner of death microbiomes, as well as a potential biomarker of infant death related to preterm birth. The methodology presented here also supports the routine collection of postmortem microbiotas from a variety of anatomical sites as an addition to existing proposed standard microbiological sampling and autopsy protocols. Additionally, this study goes beyond culture-based methods to identify potential biomarkers and consortia that may be positively correlated with sudden death in infants. These data are just one more step toward our understanding of the complex dynamics that make up the human microbiome. Characterizing postmortem microbial interactions is critical to build our knowledge base regarding human microbiome composition across a broad range of ages, lifestyles, demographics, and socio-economic status. More data are needed to develop a truly variable, representative database that can proactively inform both forensics and human health in the way antemortem microbiome studies have transformed informed decision-making guidelines.
ACKNOWLEDGMENTS
The authors acknowledge and thank the Wayne County Medical Examiner's Office personnel for their collaboration and assistance with sample collection, and the MSU Genomics Core Facility staff for their support in sequencing efforts. We also sincerely thank Kelly Waters, Anthony Grigsby, Dr. Sophie Picq, Alex Rakestraw, Emily Schnettler, and Alexandria Verner for their assistance with sample processing and technical support. Preliminary findings of this research were shared at the National Institute of Justice 2023 National Research Conference, May 24, 2023, in Arlington VA; the National Institute of Justice Forensic Science Research and Development Symposium, February 20, 2024, in Denver, CO; and at the 76th Annual Conference of the American Academy of Forensic Sciences, February 19–24, 2024, in Denver, CO.
FUNDING INFORMATION
This research was funded by a grant from the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice awarded (2020-75-CX-0012) to JLP, HRJ, MEB, and CJS. Points of view in this document are those of the authors and do not necessarily represent the official position or policies of the U.S. Department of Justice. Additional funding provided by the Michigan State University (MSU) Research Foundation, MSU colleges, and the Strategic Partnership Grants (SPG).
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interests to disclose.




