The impacts of a typical dairy cow crossbreeding strategy on Cheddar cheesemaking efficiency, nutrition and quality
Abstract
Background
Crossbreeding can be utilised in animal husbandry to increase genetic diversity and improve health and fertility in the next generation. In dairy herd management, a common strategy involves crossbreeding Jersey (JE) cows with Holstein-Friesians (HF), resulting in Jersey-Holstein-Friesian (JFX) progeny.
Aim(s)
This due-diligence study aimed to investigate the impact of these genotypes on processing efficiency and product quality within the Cheddar manufacturing process.
Methods
Cheddar cheesemaking trials were completed using HF and JFX derived milks, and the composition and yield of the resultant cheeses were determined. The ripening characteristics of these cheeses were then investigated over a six-month maturation period. The study was completed in triplicate; mid-lactation milk was collected on three occasions in August 2022, from a grazing farm systems experiment at Teagasc, Moorepark. Cows had been divided into JFX and HF groups, each group consisting of 25 cows randomised for age, yield, and parity. All were grazed on a typical pasture diet of perennial ryegrass.
Major findings
On average, raw JFX milk had significantly higher total protein (+ 5.68%) and fat (+ 13.20%) compared to HF milk. However, producing Cheddar with standardised milks resulted in no significant differences in milk coagulation properties or cheese yield. All cheese compositions were within the range of expected values for Cheddar. No meaningful differences resulted in texture, proteolysis and pH during the ripening period, and no significant differences were observed in volatile-organic-compounds and fat profiles between the HF and JFX derived cheeses.
Scientific or industrial implications
From a due-diligence perspective, it was not evident from this analysis that JFX phenotypes cause practical negative impacts on the cheesemaking ability of milk or adversely influence final cheese quality. Furthermore, cheese derived from JFX milk was more yellow in colour, with significantly higher b* values, which is indicative of preferable sensory and nutritional quality.
INTRODUCTION
In Ireland, the national dairy herd is predominantly comprised of Holstein-Friesian (HF) cows, a breed established through careful selective breeding of Holstein (HO) and Friesian (FR) animals (Evers et al. 2021). The breed is highly specialised, producing high volumes of milk with sufficient levels of protein and fat to maximise total solid yields (Palladino et al. 2010; Coffey et al. 2016). Milk fat plus protein is more important than milk volume in Ireland and Irish farmers are paid for total protein and fat yielded with a penalty for milk volume (Geary et al. 2010). European dairy systems have typically focused selective breeding efforts towards increased productivity and efficiency within the HF population (Brito et al. 2021). The approach has brought benefits, but a problematic consequence has been increased rates of inbreeding depression (Teagasc 2016; Brito et al. 2021). Breeding strategies that increase genetic diversity are therefore coming into greater focus.
Dairy cows inherit two copies of each gene, one from each parent, and inbreeding increases the likelihood that two matching gene copies will be present in the next generation. This is desirable for good genes, but over time an increase in deleterious recessive gene pairs also occurs (Cassell 2007). Repeated generations of inbreeding negatively impact herd health, causing reduced conception rates, increased stillbirth rates and reduced disease resistance (Hansen 2006). Crossbreeding can reverse inbreeding-related reductions in animal health and improve herd productivity (Buckley et al. 2014; Saha et al. 2020) because the frequency of different gene copies is increased in the next generation (Sørensen et al. 2008). Increased heterozygosity increases genetic diversity and brings hybrid vigour (Shull 1948; Teagasc 2016). The amount of hybrid vigour resulting in crossbred progeny is positively correlated with the genetic distance between parental breeds (Mäki-Tanila 2007; Ducrocq and Wiggans 2015). Coffey et al. (2016) described greater hybrid vigour for JFX offspring than HF offspring, resulting from a greater genetic distance between parental breeds. Teagasc (2012) found that JFX cows are better at maintaining body condition, have superior reproductive efficiency, and longer milking durations than HF cows. Crossbreeding has already grown in popularity in some countries. Sixty per cent of New Zealand's dairy herd are JFX crossbreeds, 25% HF, and the remainder comprises JE and other breeds (Dairynz 2022). In Ireland, HF cows dominate, and JFX cows represent around 5% of the Irish herd (Coffey et al. 2016).
Crossbreeding can improve herd health, but due consideration must also be given to the impact of crossbreeding on milk production traits and milk composition arising from such animals. Some previous research has associated crossbreeding with lower milk volumes (Anderson et al. 2007); other work suggests a neutral or positive impact on production traits (Anderson et al. 2007; Bryant et al. 2007; Teagasc 2012; Vance et al. 2012; Coffey et al. 2016). Coffey et al. (2016) found crossbreeds exceeded their respective parents in milk solid production, and the greatest milk solids were observed in JFX milk. Similarly, Anderson et al. (2007) and Bryant et al. (2007) found JFX milk had higher fat and protein contents than purebred HO milk. A Teagasc (2012) study reported lower milk volumes from JFX cows than HF, but with higher milk solids. Vance et al. (2012) found HF milking performance could be improved relative to JFX by increasing dietary concentrate levels, an interaction between genetics and diet. It should be noted that whilst hybrid vigour is relevant to milk production, heterotic impacts have been reported as being only 0–10% for milk production traits, compared with 5–25% for fertility traits (Coffey et al. 2016). Regarding the phenotypic presentation of parental genetics within progeny, this can be additive or synergistic, synergism suggesting hybrid vigour. Heterosis is observed when offspring exceed the average performance of their parents for a given trait (Ducrocq and Wiggans 2015). Palladino et al. (2010) found that JFX offspring had both protein and fat yields that exceeded parental averages; JFX milk yielded 0.69 kg/day protein, above the parental average of 0.665 kg/day, and yielded 0.81 kg/day fat, which exceeded the parental average of 0.775 kg/day.
Further to milk composition, thought should be given to milk-processing attributes; there is a paucity of information on the impacts of crossbreeding on milk processability. Purebred JE milk can provide specific benefits for cheesemaking (Auldist et al. 2004; Bland 2015; Kristensen et al. 2015; Yoo et al. 2019). Auldist et al. (2004) demonstrated better curd formation and a 10% greater cheese yield from JE milk than FR milk, attributed to higher milk solids content. Similarly, using nonstandardised milk, Bland (2015) found that JE milk resulted in a higher yield of Cheddar cheese than HF milk. Yoo et al. (2019) found that cheese made from JE milk had increased micronutrient content and improved colour, taste, flavour and texture scores, particularly in ripened cheese, compared with HO milk. Consideration should also be given to the cheese manufacturing qualities of crossbred cow milk, and whether JFX milk shares techno-functional properties with JE milk (Sneddon et al. 2015; Saha et al. 2020; Sanjayaranj et al. 2023). Saha et al. (2020) found higher casein contents and slightly improved coagulation and curd-firming properties in milk from crossbred cows compared with purebreds. Sanjayaranj et al. (2023) found a positive association between milk coagulation properties and crossbreeding but acknowledged interpretation of these results was limited by sample size, and some confounding variables also limited the usefulness of this study. Sneddon et al. (2015) observed significant positive heterosis for cheese yield; they reported that JFX cows should yield 25.4 kg more cheese over a lactation than HF cows.
Teagasc (2012) stated that JFX cows could increase profits on Irish dairy farms by €20 000/year within a 40-h unit. This potential for improved economic sustainability and other aforementioned evidence provide justification to consider additional use of crossbreeding within dairy food systems. Approximately one-third of Irish milk is destined for cheese production (Fox et al. 2017), and investigations into cheese quality utilising JFX milk are under-reported in the literature. Consequently, this study aimed to investigate the effects of JFX crossbreeding on Cheddar cheese produced from an Irish pasture-grazing system.
MATERIALS AND METHODS
Milking systems and milk collection
The milk used within this study was supplied by the Animal and Grassland Research and Innovation Centre, Teagasc, Moorepark. The work utilised mid-lactation milk collected on three occasions in August 2022, from a grazing farm systems experiment undertaken by Jezequel et al. (2024). Cows were grazed in two herds on a typical Irish pasture diet of monoculture swards of Lolium perenne [perennial ryegrass (PRG)]. The treatment groups comprised Holstein-Friesian (HF) and Holstein-Friesian × Jersey (JFX) crossbred animals, with 25 cows per group randomised for age, breed, yield and parity. The average economic breeding index (EBI), milk, fertility, calving, beef, maintenance, management and health sub-indices of the HF cows was €205, 63, 103, 33, −12, 15, 2 and −1, respectively, and €198, 68, 89, 30, −24, 32, 3 and −1, respectively, for the JFX cows. The average EBI of all cows ranked them in the top 1% of the national herd during the experimental period. All animals were milked twice daily at 6.30 AM and 4.30 PM. Both herds grazed on a rotational basis adjacent to one another, separated using a temporary electric fence to ensure diets of similar quality were offered. Pre-grazing yield, daily herbage allocation and post-grazing residual heights were similar between treatment groups [1650 ± 28.9 kg (DM/ha >4 cm), 15.5 kg DM/ha, 40 mm, respectively]. Sward composition was 20.5 ± 1.50% (% crude protein) with dry matter digestibility of 80.1 ± 3.3% (Jezequel et al. 2024). The cows were offered 1 kg of concentrate at each milking (overall 2 kg/day). Further details can be found in Jezequel et al. (2024) which describes the detailed grazing characteristics and general animal performances during the study period. Raw milk for both treatment groups was collected on the same day between 8:30 AM and 9:00 AM. This raw milk was then stored overnight at 4°C, ready for the preparation of the milk for cheese production, beginning the next day.
Preparation of cheesemilk
On the day following milk collection, the standardisation and pasteurisation of milks were completed for both treatment types; all milks within a given trial underwent preparation for cheesemaking at the same time and under the same conditions. A portion of the collected raw milk, ~ 5 kg, was separated into cream and skim milk at 50°C using a benchtop centrifuge operating at 10 000 revolutions per minute (FT15 Disc Bowl Centrifuge; Armfield Limited, Hampshire, UK). Milk from each crossbreeding treatment was then standardised to a total protein-to-fat ratio (Guinee et al. 2006) of 0.95 using skim milk, raw whole milk or cream. These standardised milks were then pasteurised at 72°C for 15 s (HTST/UHT System HT122; OMVE, Scgalkwijk, the Netherlands). Milk that has been standardised and pasteurised will be referred to as cheesemilk within this study. All cheesemilks were then stored overnight at 4°C, ready for the Cheddar cheese manufacturing process which would commence the following morning.
Cheese manufacturing
Within a given cheese manufacturing trial, all Cheddar produced within that trial, for both treatment types, was manufactured side by side, and under the same production conditions, on the same day. On the day following standardisation and pasteurisation, Cheddar cheese was manufactured utilising pilot-scale production vats (10 L; Pierre Guerin Technologies, Mauze, France) at the Biofunctional Food Engineering Facility, Teagasc Food Research Centre, Moorepark, Fermoy, Co Cork, Ireland, as described by Page et al. (2024). Briefly, 12 kg of cheesemilk per vat was heated to 32°C and the pH was adjusted to 6.55 using a 4% (v/v) lactic acid solution. A frozen starter culture, comprising Lactococcus lactis subsp. cremoris and Lactococcus lactis subsp. lactis, was added at a level of 2.2 g per 12 kg cheesemilk (R604; Chr. Hansen Ireland Ltd., Co. Cork, Ireland). After 20 minutes, fermentation-produced calf chymosin was added to each vat [0.18 mL per kg of milk, diluted 1:10 prior to addition, CHY-MAX, 200 international milk clotting units (IMCU)/mL; Chr. Hansen Ireland Ltd., Co. Cork, Ireland]. Gels were then cut at a constant firmness (G′) value of 35 Pa (Anton Paar, Graz, Austria). Curds were cooked to 38°C at a rate of 0.25°C/minute, drained at pH 6.15, milled at pH 5.35, salted at 2.7% (w/w), mellowed for 20 minutes, moulded and pressed overnight. After manufacturing, all cheeses were vacuum packed and ripened at 8°C for 6 months (180 days).
Cheddar cheese manufacturing trials were completed in triplicate in August 2022; this means that the entire end-to-end cheesemaking process, as well as the process of milk collection and standardisation described above, were each performed in full on three separate occasions.
Chymosin coagulation properties
Samples were taken to determine the coagulation properties of HF and JFX cheesemilks according to the method described by Page et al. (2024). Milk samples were removed from cheese vats 3 min after chymosin addition and stirring, and then placed into the cell of a small amplitude oscillatory rheometer (Physica MCR 501, Anton Paar, Graz, Austria), utilising a concentric-cylinder measuring geometry. The dynamic changes in rheological properties during the coagulation process were monitored using a dynamic time sweep analysis with an angular frequency of 1.0 Hz and a strain of 0.01 at 32°C, within the linear viscoelastic region (strain <0.03) reported for chymosin milk gels. The time from chymosin addition to reaching a G′ value of 35 Pa was used as set-to-cut time (time from chymosin addition to cutting) within the cheese manufacturing process (Lamichhane and Sheehan 2018a). Chymosin coagulation time (RCT) was calculated by measuring the time taken from chymosin addition until a storage modulus of 1 Pa was reached.
Composition of raw milk, cheesemilk, whey and cheese
Fat, protein and lactose contents in raw milk were determined within the milk standardisation process through a milk analyser using FTIR spectroscopy (DairySpec FT; Bentley Instruments Inc., Minnesota, USA). The cheesemilk and bulk whey samples were analysed for total solids using the oven-drying method (IDF 2010). Bulk whey is defined as whey collected during whey drainage and curd cheddaring (Guinee et al. 2006). Fat and total protein contents in cheesemilk and whey were determined with the Rose-Gottlieb method (IDF 1987) and the Kjeldahl method (IDF 2014), respectively. A nitrogen-to-milk protein conversion factor of 6.38 was used. Nonprotein nitrogen (NPN) and noncasein nitrogen (NCN) levels in cheesemilks were determined as described by Xia et al. (2020). Somatic cell counts (SSC) in cheesemilks were measured using a fluoro-opto-electronic counter (Fossomatic FC, Foss, Hillerød, Denmark). The levels of calcium, potassium, magnesium, sodium, phosphorus and zinc were determined in milk, bulk whey and cheese using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES; Agilent 5110, California, USA). The determination of mineral content was carried out using standard International Dairy Federation (IDF) methodology as described in IDF (ISO/IDF229:2018 2018) and Agilent application note 15 151 (Agilent 2023). Samples were digested using a microwave digestion system (CEM Mars 6; CEM Corporation, NC, USA) as described by Deshwal et al. (2023). Grated cheese samples were analysed in duplicate at 21 days of maturation for moisture, fat and protein contents, as described by Xia et al. (2020).
Cheese yield
Actual cheese yield (Ya), moisture-adjusted cheese yield (Yma) and moisture-adjusted cheese yield with fat and protein adjusted to reference levels (Ymafpam) were calculated as described by Guinee et al. (2006). The reference moisture for the determination of moisture-adjusted cheese yield (Yma) was 38.5% (w/w).
Analysis of total fatty acids, free fatty acids and volatile compounds
The total fatty acid (TFA), free fatty acid (FFA) and volatile compound levels were determined in cheese at 180 days of maturation. The TFA profile of cheese and milk was determined according to the method described in O'Callaghan et al. (2020). The FFA profile of cheese samples was determined according to the methods of De Jong and Badings (1990) with modifications as outlined in Mannion et al. (2016). The volatile compounds were determined using gas chromatography–mass spectrometry (GC–MS) as described by Lamichhane and Sheehan (2018a).
Texture profile analysis
Texture profile analysis was performed using TA.XTplus Texture Analyser (Stable Micro Systems, Surrey, UK) equipped with a 70 mm (diameter) compression plate and a 50 kg load cell. Three cube-shaped cheese samples (2.5 cm3) were removed from the centre of each cheese type with a wire cutter, tightly wrapped in aluminium foil and refrigerated for at least 5 h, sufficient time to make certain that the internal cheese temperature had dropped from around 8–4°C. Samples were then individually removed from 4°C storage just prior to immediate testing taking place; the sample temperature for the purposes of each and all individual tests was therefore ~4°C. Cheese cubes were compressed to 70% of original height in two successive bites at a rate of 60 mm/min. This analysis was repeated at Days 1, 21, 90 and 180 over-maturation. The texture parameters were determined using Texture Expert Exceed software.
Colour analysis
Colourimetric analysis was performed according to the Commission Internationale de l'Eclairage, or, CIELAB colorspace approach. Measurements were made on the internal surfaces of the cheese with freshly cut samples taken at 1, 21, 90 and 180 days of maturation. The colour measurements were performed using a colorimeter (CR-400; Konica Minolta, NJ, USA).
pH and proteolysis
The pH was measured in cheese samples at Days 1, 21, 90 and 180 of maturation. A cheese slurry was prepared with 20 g of fresh ground cheese and 12 g of de-ionised water. The pH was recorded using a bench pH meter. To measure the relative extent of casein degradation between HF and JFX cheeses, the levels of nitrogen soluble (expressed as % of total nitrogen) at pH 4.6 (pH 4.6-SN/TN) were also measured at Days 1, 21, 90 and 180 of over-maturation as described by Fenelon and Guinee (2000).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 27 (IBM Statistics Inc.), with some processing and formatting of data carried in Microsoft Excel 64 bit, 2016 Version (Microsoft Corporation Inc.) and GraphPad Prism version 9 (GraphPad Software Inc.). Univariate ANOVA was used for the comparison of means in single factors, and repeated measures ANOVA was used to assess for statistical significance in data related to changes in cheese during the maturation period. For all statistical analyses, P values <0.05 were considered significant. The reported data are the average of data taken from three separate cheesemaking trials, with results presented as the mean ± one standard deviation.
RESULTS AND DISCUSSION
Raw milk, cheesemilk and whey composition
The raw milk and cheesemilk compositions of HF and JFX milk are shown in Table 1. The JFX raw milk had significantly higher levels of protein and fat than HF raw milk (P < 0.05), and similar results for increased fat and protein in JFX milk have been reported previously (Anderson et al. 2007; Bryant et al. 2007; Teagasc 2012; Coffey et al. 2016). No significant effects resulting from the crossbreeding factor were observed for lactose content (P > 0.05). Similar to raw milk, cheesemilk had significantly higher levels of protein and fat than HF milk (P < 0.05). No significant effects resulting from the crossbreeding factor were observed in cheesemilk for total solids, lactose content, nonprotein-nitrogen, casein number and casein content (P > 0.05).
| HF | JFX | P-value | |
|---|---|---|---|
| Raw milk composition | |||
| Total protein (% wt/wt) | 3.52 ± 0.11 | 3.72 ± 0.03 | * |
| Fat (% wt/wt) | 4.62 ± 0.22 | 5.23 ± 0.32 | * |
| Lactose (% wt/wt) | 4.68 ± 0.11 | 4.57 ± 0.16 | NS |
| Cheesemilk composition | |||
| Total protein (% wt/wt) | 3.55 ± 0.13 | 3.78 ± 0.02 | * |
| True protein (% wt/wt) | 3.38 ± 0.12 | 3.61 ± 0.02 | * |
| Fat (% wt/wt) | 3.74 ± 0.13 | 3.99 ± 0.04 | * |
| Lactose (% wt/wt) | 4.67 ± 0.10 | 4.59 ± 0.18 | NS |
| Total solids (%) | 12.94 ± 0.43 | 13.79 ± 0.42 | NS |
| Nonprotein nitrogen (% wt/wt) | 4.75 ± 0.64 | 4.51 ± 0.10 | NS |
| Casein number | 79.00 ± 0.69 | 78.28 ± 0.40 | NS |
| Casein content (% wt/wt) | 2.80 ± 0.12 | 2.96 ± 0.01 | NS |
| Calcium (mg/kg) | 1515 ± 128 | 1421 ± 87 | NS |
| Potassium (mg/kg) | 1524 ± 58 | 1489 ± 64 | NS |
| Magnesium (mg/kg) | 110 ± 16 | 128 ± 10 | NS |
| Sodium (mg/kg) | 418 ± 64 | 382 ± 31 | NS |
| Phosphorous (mg/kg) | 1117 ± 119 | 1028 ± 75 | NS |
| Zinc (mg/kg) | 5.09 ± 0.29 | 4.74 ± 0.28 | NS |
| Somatic cell count (SCC) | 131 ± 67 | 222 ± 136 | NS |
| Whey composition | |||
| Weight of whey (kg) | 10.41 ± 0.02 | 10.26 ± 0.04 | * |
| Total solids (%) | 7.20 ± 0.22 | 7.10 ± 0.29 | NS |
| Protein (% wt/wt) | 0.95 ± 0.04 | 1.12 ± 0.12 | NS |
| Fat (% wt/wt) | 0.40 ± 0.04 | 0.38 ± 0.01 | NS |
| Fat lost in whey (%) | 9.33 ± 1.56 | 8.20 ± 0.22 | NS |
- Data are presented as mean ± standard deviation.
- *P < 0.05, NSP > 0.05 (non-significant).
The mineral contents of both cheesemilks were within the range of expected values; they were similar to previously reported data from other investigations. Additionally, no statistically significant differences were found in milk mineral composition as a result of the crossbreeding treatment (P > 0.05). Levels of calcium, potassium, sodium, phosphorus and zinc were marginally higher in HF milk, and levels of magnesium were slightly higher in JFX milk, but none of these differences were statistically significant (P > 0.05). The mineral content of milk is important nutritionally and in terms of functional properties. From a micronutrient perspective, dairy products provide meaningful contributions of minerals to Irish diets (Feeney et al. 2016). Mineral levels can impact the functional performance of milk during processing with relevance to the cheese manufacturing process (Lamichhane and Sheehan 2018b). Lamichhane and Sheehan (2018b) described how the mineral contents of milk, particularly calcium and phosphate, can impact upon myriad aspects of final cheese quality. Indeed, the calcium and phosphate associated with casein have a structural role in Cheddar cheese and deviations in these minerals can impact cheese texture (O'Mahony et al. 2005). Overall, no significant differences in mineral composition between the treatment groups were found, and this suggests that crossbreeding might be applied without any adverse mineral-related nutritional and processing impacts.
This research found no significant association between SCC and crossbreeding. The SCC values were higher in JFX milk, albeit not significantly (P > 0.05). Previous work has found significantly higher SCC for JFX milk (Coffey et al. 2016). In other work, reduced SCCs associated with the crossbreeding of dairy cows have been described as being the result of hybrid-vigour related effects (Saha et al. 2020).
Regarding the whey obtained during cheese manufacturing, the JFX treatment did result in a significantly lower amount of bulk whey than HF (P < 0.05), 10.26 kg compared with 10.41 kg, respectively, and this difference in whey volume is consistent with the greater amount of cheese yielded from the JFX crossbreeding treatment. Regarding the composition of bulk whey, any differences observed between the fat, protein and total solids contents were not significant differences (P > 0.05) (Table 1). The percentage of fat from cheesemilk lost into the whey was greater for HF than for JFX, but this difference was not statistically significant (P > 0.05). Overall, it is not evident through this study that the crossbreeding factor significantly impacts the components lost into the whey.
Cheese composition and cheese yield
The protein, fat and moisture percentages in cheese were consistent with previously reported observations for standard full-fat Cheddar cheese (Feeney et al. 2021) (Table 2). In addition, no statistically significant differences were observed between HF and JFX Cheddar for protein, fat and moisture. Likewise, fat-in-dry matter (FDM), moisture in nonfat substance (MNFS), salt content and salt-in-moisture (S/M) also showed no significant differences between the crossbreeding treatments (P > 0.05).
| HF | JFX | P-value | |
|---|---|---|---|
| Protein (% wt/wt) | 26.87 ± 0.68 | 27.27 ± 0.61 | NS |
| Fat (% wt/wt) | 33.83 ± 0.57 | 32.65 ± 0.58 | NS |
| Moisture (% wt/wt) | 34.42 ± 1.14 | 35.79 ± 0.37 | NS |
| FDM (% wt/wt)a | 51.59 ± 0.2 | 50.84 ± 0.61 | NS |
| MNFS (% wt/wt)b | 52.02 ± 1.29 | 53.14 ± 0.13 | NS |
| Salt (% wt/wt) | 1.63 ± 0.19 | 1.64 ± 0.08 | NS |
| S/M (% wt/wt)c | 4.74 ± 0.54 | 4.58 ± 0.22 | NS |
| Actual yield (Ya) | 10.05 ± 0.43 | 10.36 ± 0.35 | NS |
| MACY (Yma)d | 10.72 ± 0.51 | 10.82 ± 0.4 | NS |
| MACYPFAM (Ymafpam)e | 9.85 ± 0.13 | 9.34 ± 0.42 | NS |
| Calcium (mg/kg) | 8977 ± 818 | 8381 ± 569 | NS |
| Potassium (mg/kg) | 732 ± 49 | 678 ± 38 | NS |
| Magnesium (mg/kg) | 347 ± 45 | 342 ± 29 | NS |
| Phosphorous (mg/kg) | 5880 ± 416 | 5564 ± 325 | NS |
| Sodium (mg/kg) | 6179 ± 498 | 6364 ± 570 | NS |
| Zinc (mg/kg) | 42 ± 3 | 39 ± 1.1 | NS |
- Data are presented as mean ± standard deviation.
- a Fat in dry matter.
- b Moisture in non-fat substance.
- c Salt in moisture.
- d Moisture adjusted cheese yield.
- e Moisture adjusted cheese yield adjusted for protein and fat.
- NSP > 0.05 (non-significant).
Regarding cheesemaking efficiency, no statistically significant differences were observed in actual yield (Ya), moisture-adjusted cheese yield (Yma) or moisture-adjusted cheese yield adjusted for protein and fat (Ymafpam)(P > 0.05). At the very least, cheese manufacturers should be reassured by this evidence; within this work, the use of JFX milk did not reduce cheese yield, and the macro-composition was not adversely impacted by crossbreeding. It should, however, be noted that JFX milk did on average result in a higher Ya of 10.36 than of 10.05 for HF milk (Table 2), a 3.14% higher yield. This higher cheese yield from JFX cheesemilk, compared with HF cheesemilk, is attributed to its naturally higher protein content (Table 1). However, when moisture is considered, the cheese yield differences between the JFX and HF breeds are much less apparent; the Yma values for JFX and HF were 10.82 and 10.72, respectively. Previous work has suggested the possibility of improved cheese yield from JFX milk. This included milk with significantly increased protein and fat yield from crossbreeds (Coffey et al. 2016) and JFX milk having a significantly increased milk solids yield (Teagasc 2012). Indeed, significantly higher protein and fat levels were also found in JFX milk within this work, and previous studies have shown that milk with higher protein and fat levels typically results in a higher cheese yield (Guinee et al. 2006; Page et al. 2024). In a New Zealand-focused study, JFX milk was suggested to yield significantly more cheese than HF milk (Sneddon et al. 2015); JFX milk was suggested to yield 6.5% more cheese than HF milk, with average yields of 12.4% and 11.64%, respectively. However, the work of Sneddon et al. (2015) was completed on data from New Zealand, and many differences could be present within a study focused within a different location, so care does need to be taken when comparing between studies. Indeed, crossbreeding effects are known to be mediated by interactions with the environment, the exact diet the cows are given, and the farming system applied (Vance et al. 2012). In addition, experimental methods differed substantially; this current study was a triplicate cheese manufacturing study completed at mid-lactation, whilst the significant differences in cheese yield suggested by Sneddon et al. (2015) utilised data from a mass-balance milk-processing model applied over an entire lactation; Sneddon et al. (2015) relied on predicted cheese yields obtained from compositional data, allowing investigation of a much larger data set, but without having actually conducted any cheesemaking trials.
Regarding the mineral composition of cheese, no significant effects were observed as a result of the crossbreeding treatment (P > 0.05). Similar to cheesemilk mineral data, calcium, potassium, zinc and phosphorous contents were also higher in HF cheese, but again these differences were not statistically significant (P > 0.05). The magnesium content of HF milk was slightly higher than JFX milk, but the magnesium content was higher in JFX cheese. These differences in magnesium are unlikely to be meaningful; they were not statistically significant, and the magnitude of the differences was also particularly small. Regarding mineral content, the overriding suggestion is that mineral composition appears to be largely unaffected in cheese manufactured from JFX milk compared with HF milk. However, some low-magnitude and nonsignificant differences are present, and within these differences, HF milk and cheese did repeatedly tend to show a pattern of higher mineral levels.
Rheological properties of coagulated cheesemilk
No significant differences in rheological properties were observed between JFX and HF cheesemilks (P > 0.05). The average set-to-cut time was 28.97 ± 3.5 min for JFX cheesemilk compared with a slower 33.63 ± 3.8 min for HF cheesemilk, but with a high degree of variation in this property, these differences were not statistically significant (P > 0.05). The protein content of milk impacts upon chymosin gelation properties, and the faster set-to-cut time of JFX cheesemilk was most likely due to this milk having a slightly higher protein content than HF cheesemilk (Table 1). The casein content of JFX milk was also higher than HF milk, albeit not significantly (P > 0.05). The average chymosin coagulation time (RCT) was also not significantly different between the two breed types (P > 0.05) at 17.37 ± 2.1 min for JFX cheesemilk and 18.43 ± 0.6 min for HF cheesemilk.
Overall, milk gelation properties appear to be not adversely impacted by this crossbreeding strategy. The average set-to-cut time was ~5 min faster for JFX milk, and whilst this was not a statistically significant result, this observation might still indicate a pattern for JFX milk compared with HF milk. However, it is worth noting that in commercial operations with automated vat systems, slight adjustments to the cutting time could be required when transitioning from HF milk to JFX milk. Consequently, additional research into the rheological properties of JFX milk might be a consideration for future investigators, in particular, giving consideration to this property at a commercial scale, given this study was completed at pilot scale. If this magnitude of rheological difference was repeatable in a larger study, it could be practically meaningful to manufacturers. Furthermore, some other research studies have observed statistically significant associations between milk coagulation and curd-firming properties when utilising milk obtained from crossbred dairy cows and have additionally described the link between these factors and increased protein or casein contents (Saha et al. 2020; Sanjayaranj et al. 2023).
pH of cheese during the maturation period
No significant differences in cheese pH values were observed during the maturation period in response to the crossbreeding factor (Table 3). The pH values are in the range of 5.10–5.37, which is typical of Cheddar cheese. Overall, the pH of all cheese increased significantly as it ripened (P < 0.05). Change in pH with time is likely due to a number of factors, which include the proteolytic liberation of ammonia, free basic amino acids and amines (Lamichhane and Sheehan 2018a), and the solubilisation of calcium associated with casein (Hassan et al. 2004).
| 1 Day | 21 Days | 90 Days | 180 Days | Treatment P-value | Time P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HF | JFX | HF | JFX | HF | JFX | HF | JFX | |||
| pH development | ||||||||||
| pH | 5.10 ± 0.04 | 5.09 ± 0.04 | 5.21 ± 0.06 | 5.22 ± 0.04 | 5.30 ± 0.03 | 5.33 ± 0.02 | 5.28 ± 0.03 | 5.37 ± 0.05 | NS | * |
| Texture profile analysis | ||||||||||
| Fracturability (N) | 415 ± 30 | 434 ± 55 | 314 ± 33 | 327 ± 32 | 259 ± 32 | 264 ± 11 | 222 ± 17 | 239 ± 23 | NS | * |
| Hardness (N) | 373 ± 11 | 365 ± 36 | 319 ± 84 | 344 ± 22 | 310 ± 19 | 335 ± 33 | 284 ± 13 | 286 ± 20 | NS | * |
| Resilience (%) | 8.33 ± 0.85 | 6.81 ± 2.52 | 6.60 ± 0.18 | 6.76 ± 0.80 | 5.92 ± 0.25 | 6.40 ± 1.12 | 5.10 ± 0.48 | 4.69 ± 0.37 | NS | * |
| Cohesiveness (unitless) | 0.210 ± 0.03 | 0.192 ± 0.04 | 0.200 ± 0.02 | 0.201 ± 0.02 | 0.199 ± 0.01 | 0.213 ± 0.02 | 0.169 ± 0.01 | 0.158 ± 0.01 | NS | * |
| Springiness (%) | 85.31 ± 27.61 | 78.92 ± 8.80 | 51.74 ± 5.51 | 51.27 ± 3.47 | 40.17 ± 4.58 | 38.9 ± 8.67 | 35.16 ± 7.16 | 35.52 ± 7.24 | NS | * |
| Proteolysis | ||||||||||
| pH 4.6 SN/TNa | 2.44 ± 0.10 | 2.26 ± 0.08 | 4.85 ± 0.58 | 5.09 ± 0.59 | 10.78 ± 0.36 | 11.17 ± 0.38 | 14.83 ± 1.45 | 15.33 ± 0.79 | NS | * |
| Colour | ||||||||||
| L* | 86.53 ± 1.93 | 87.89 ± 1.22 | 78.65 ± 0.54 | 76.56 ± 1.60 | 77.64 ± 1.59 | 77.37 ± 1.16 | 78.76 ± 1.81 | 78.01 ± 1.03 | NS | * |
| a* | −4.36 ± 0.35 | −4.43 ± 0.46 | −5.25 ± 0.23 | −5.36 ± 0.27 | −5.15 ± 0.40 | −5.33 ± 0.43 | −4.70 ± 0.27 | −5.03 ± 0.17 | NS | * |
| b* | 27.04 ± 2.03 | 30.35 ± 1.18 | 32.01 ± 1.03 | 34.75 ± 1.69 | 32.20 ± 1.14 | 35.21 ± 1.70 | 30.68 ± 1.29 | 34.19 ± 1.07 | * | * |
- a Nitrogen soluble at pH 4.6 as a percentage of total nitrogen.
- *P < 0.05 (significant), NSP > 0.05 (non-significant).
Proteolysis of cheese during the maturation period
The extent of primary proteolysis within all cheese investigated was consistent with previously reported ranges of results (Fenelon and Guinee 2000; Page et al. 2024). Primary proteolysis is expressed as a percentage of soluble nitrogen at pH 4.6 as a percentage of total nitrogen (pH 4.6-SN/TN) (Table 3). Primary proteolysis increased significantly with maturation time (P < 0.05), attributed to the action of residual chymosin, plasmin and other enzymes present from starter and nonstarter bacteria. Importantly, no significant differences in primary proteolysis were observed between the breed treatment types (P > 0.05), and proteolysis has an important role in the texture development of cheese (Fox 1989).
Cheese texture during the maturation period
The data obtained within the texture development experiments (Table 3) are supported by the previously discussed primary proteolysis analysis; the results obtained by these different experimental approaches provide further validation of the findings. No significant impacts were observed between HF and JFX cheese for the texture attributes of fracturability, hardness, resilience, cohesiveness and springiness (P > 0.05) (Table 3). All of the investigated texture parameters decreased significantly over-maturation (P < 0.05). This type of maturation-related change within cheese is to be expected, resulting from factors, such as proteolysis, pH and solubilisation of colloidal calcium, and similar results have already been reported previously (O'Callaghan et al. 2017; Lamichhane and Sheehan 2018a; Lamichhane et al. 2019).
Cheese colour during the maturation period
The crossbreeding factor had a significant impact on the colour attributes of Cheddar cheese, specifically through significantly higher b* values (P < 0.05), whilst L* and a* values did not show significant differences (Table 3; Figure 1). The b* parameter references blue to yellow colour, and the significantly higher b* values indicate that JFX cheese is more yellow in colour than HF cheese. The JFX cheese also appeared visually more yellow in colour when compared to HF cheese. Yellow colouring of dairy products has an association with pasture-grazing, and a positive correlation between colour scores awarded by sensory panellists and b* values of Cheddar has already been described in previous studies (O'Callaghan et al. 2017). However, it should also be acknowledged that sensory preferences can differ between geographical locations (Ouyang et al. 2021). Further to consumer choice, yellow colouring of milk and cheese does stem from a legitimate nutritional and health association, particularly when yellow colouring results from β-carotene which is both a pigment and a pro-vitamin (O'Callaghan et al. 2017; Page et al. 2024). Considering the β-carotene content of cheese, Lucas et al. (2006) found that β-carotene is transferred efficiently from milk to cheese during manufacturing, at an average rate of 950 g/kg. Consequently, when differences in β-carotene arise in milk these differences should also be apparent in the resultant cheese. Within this work, significant differences in yellow colour were seen between JFX and HF Cheddar, and other investigators have also described how β-Carotene concentration in milk can vary substantially between dairy cow breeds (Jensen et al. 1999; Morris et al. 2002; Nozière et al. 2006). Jensen et al. (1999) suggested that β-carotene transfers from the blood of the dairy cow to milk through an active transport mechanism and, as a consequence of this mechanism, only a genetically pre-determined maximum daily limit of β-carotene can be secreted into the milk. Morris et al. (2002) described a positive correlation between the amount of carotenoids present in milk and the ratio of JE genes compared with FR genes possessed by dairy cows. Overall, based on this discussion of the evidence, it seems likely that the higher b* values observed here for JFX Cheddar are probably indicative of a higher β-carotene content, and that this higher β-carotene content probably arises as a result of JE associated genes present in JFX cows that are either not present, or present at a lower frequency, in HF cows.
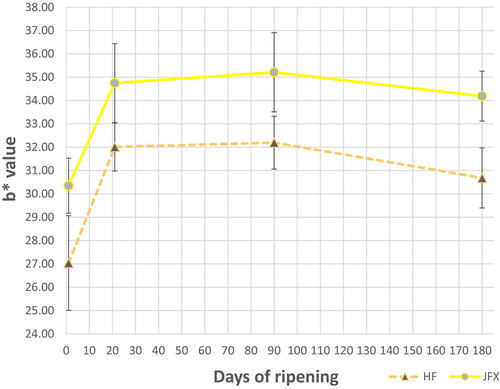
 , JFX) on the b* values of cheeses during maturation. Presented values are the means from 3 replicate cheese trials; error bars show standard deviations of the means.
, JFX) on the b* values of cheeses during maturation. Presented values are the means from 3 replicate cheese trials; error bars show standard deviations of the means.Maturation time also had a significant effect on cheese colour (P < 0.05) (Table 3). The L* parameter decreased significantly during maturation; this parameter describes how the cheese got darker as it matured. The parameter a* also changed significantly with time; this indicates a slight increase in red tone as the cheese matured. The b* values also increased significantly with maturation time (Figure 1); overall, all cheese became visually more yellow as it ripened. However, this trend mainly occurred up until 90 days of maturation, and then a slight decrease in yellow colour, red colour and darkness took place after the 90 day measurement.
Milk and cheese total fatty acid profile
The fats present in dairy products provide a meaningful contribution to diets, and the specific profile of fats that are present in milk and cheese has relevance to public health (Feeney et al. 2016). Within this research, no significant differences in total fatty acid (TFA) profile were observed between HF and JFX cheesemilk (P > 0.05) (Table 4). As this crossbreeding strategy did not significantly impact the types of fatty acids present in milk, it might also be suggested that the nutrition and health implications associated with fatty acid profile are also not meaningfully changed by crossbreeding. However, other research has found significant links between crossbreeding strategies and the fatty acid profiles of milk and dairy products (White et al. 2001; Palladino et al. 2010; Vance et al. 2012), but a lot of variation and contradiction can be seen when comparing studies. For example, Vance et al. (2012) reported that HF milk had significantly higher C14:1, C16:1 and C18:1 and higher conjugated linoleic acid (CLA) content than JFX milk, whilst JFX milk had greater levels of C18:0. They also found short-chain fatty acids (C4–C14) and saturated fatty acid (SFA) C16:0 to be unaffected by breed. Vance et al. (2012) qualified their interpretation of their findings by acknowledging and describing multiple contradictory results from other studies. Taking just one example, Vance et al. (2012) described how the work of White et al. (2001) was in agreement with their results regarding a lack of significant differences in C16:0 content, but the results of Palladino et al. (2010) contradicted this with a significantly higher C16:0 content in JFX milk. Such conflicting results are problematic and have real-world relevance, given considerable interest in dietary SFA from a nutrition and health perspective. For example, Feeney et al. (2016) described how dairy products provide a significant proportion of dietary SFA, with possible implications for cardiovascular health. Ultimately, Vance et al. (2012) conclude that variation between individual animals might be of greater importance to TFA profiles than associations linked to breed categories. Such an argument is probably relevant here too. The lack of observance of any statistically significant differences within these investigations seems reasonable within a paradigm where variation at the level of the individual cow is significantly large that it is more influential than any variation present at the level of breed.
| Milk total fatty acids | Cheese total fatty acids | ||||||
|---|---|---|---|---|---|---|---|
| HF | JFX | P-value | HF | JFX | P-value | ||
| C4:0 | 3.557 ± 0.301 | 2.843 ± 0.321 | NS | C4:0 | 4.223 ± 0.413 | 4.870 ± 0.250 | NS |
| C6:0 | 2.040 ± 0.024 | 1.857 ± 0.053 | NS | C6:0 | 2.817 ± 0.330 | 2.930 ± 0.401 | NS |
| C8:0 | 1.283 ± 0.074 | 1.240 ± 0.042 | NS | C8:0 | 1.820 ± 0.190 | 1.780 ± 0.356 | NS |
| C10:0 | 3.037 ± 0.284 | 3.030 ± 0.202 | NS | C10:0 | 4.237 ± 0.306 | 4.090 ± 0.894 | NS |
| C11:0 | 0.067 ± 0.012 | 0.060 ± 0.008 | NS | C11:0 | 0.073 ± 0.012 | 0.087 ± 0.033 | NS |
| C12:0 | 3.633 ± 0.446 | 3.640 ± 0.291 | NS | C12:0 | 4.740 ± 0.254 | 4.493 ± 0.930 | NS |
| C13:0 | 0.100 ± 0.022 | 0.090 ± 0.014 | NS | C13:0 | 0.113 ± 0.012 | 0.123 ± 0.034 | NS |
| C14:0 | 10.833 ± 1.007 | 11.097 ± 0.520 | NS | C14:0 | 13.073 ± 0.414 | 12.350 ± 1.205 | NS |
| C14:1 | 1.057 ± 0.175 | 1.047 ± 0.154 | NS | C14:1 | 1.303 ± 0.156 | 1.067 ± 0.165 | NS |
| C15:0 | 1.260 ± 0.201 | 1.227 ± 0.126 | NS | C15:0 | 1.330 ± 0.094 | 1.323 ± 0.138 | NS |
| C16:0 | 29.307 ± 1.824 | 29.743 ± 1.138 | NS | C16:0 | 30.580 ± 0.296 | 31.190 ± 0.439 | NS |
| C16:1 | 1.737 ± 0.159 | 1.587 ± 0.307 | NS | C16:1 | 1.807 ± 0.082 | 1.583 ± 0.091 | NS |
| C17:0 | 0.563 ± 0.033 | 0.557 ± 0.026 | NS | C17:0 | 0.573 ± 0.038 | 0.580 ± 0.050 | NS |
| C17:1 | 0.043 ± 0.005 | 0.030 ± 0.022 | NS | C17:1 | 0.037 ± 0.005 | 0.040 ± 0.008 | NS |
| C18:0 | 9.677 ± 1.240 | 9.637 ± 1.108 | NS | C18:0 | 8.210 ± 0.679 | 9.043 ± 1.163 | NS |
| C18:1 TFA | 3.497 ± 0.365 | 3.503 ± 0.340 | NS | C18:1 TFA | 2.400 ± 0.382 | 2.333 ± 0.078 | NS |
| C18:1n9c | 18.553 ± 1.372 | 18.460 ± 0.738 | NS | C18:1n9c | 16.153 ± 0.996 | 15.287 ± 1.833 | NS |
| C18:1n9t | 0.193 ± 0.005 | 0.167 ± 0.024 | NS | C18:1n9t | 0.140 ± 0.014 | 0.140 ± 0.022 | NS |
| C18:2 TFA | 1.803 ± 0.477 | 1.537 ± 0.266 | NS | C18:2 TFA | 0.820 ± 0.147 | 0.797 ± 0.045 | NS |
| C18:2n6c | 1.263 ± 0.017 | 1.427 ± 0.290 | NS | C18:2n6c | 0.817 ± 0.042 | 0.810 ± 0.112 | NS |
| C18:2n6t | 0.123 ± 0.017 | 0.290 ± 0.335 | NS | C18:2n6t | 0.080 ± 0.042 | 0.103 ± 0.029 | NS |
| CLA c9t11 | 1.177 ± 0.261 | 1.093 ± 0.232 | NS | CLA c9t11 | 0.863 ± 0.107 | 0.733 ± 0.025 | NS |
| C18:3 TFA | 0.317 ± 0.097 | 0.317 ± 0.097 | NS | C18:3 TFA | 0.107 ± 0.019 | 0.123 ± 0.026 | NS |
| C18:3n3 | 0.877 ± 0.093 | 1.037 ± 0.246 | NS | C18:3n3 | 0.683 ± 0.061 | 0.717 ± 0.045 | NS |
| C20:0 | 0.123 ± 0.017 | 0.133 ± 0.012 | NS | C20:0 | 0.090 ± 0.008 | 0.113 ± 0.040 | NS |
| C20:1 | 0.037 ± 0.005 | 0.023 ± 0.017 | NS | C20:1 | 0.030 ± 0.000 | 0.030 ± 0.008 | NS |
| C20:3n6 | 0.063 ± 0.005 | 0.043 ± 0.033 | NS | C20:3n6 | 0.030 ± 0.000 | 0.020 ± 0.008 | NS |
| C20:4n6 | 0.073 ± 0.009 | 0.057 ± 0.017 | NS | C20:4n6 | 0.033 ± 0.005 | 0.057 ± 0.024 | NS |
| C20:5 | 0.087 ± 0.009 | 0.087 ± 0.005 | NS | C20:5 | 0.070 ± 0.000 | 0.077 ± 0.009 | NS |
| C21:0 | 0.050 ± 0.008 | 0.023 ± 0.026 | NS | C21:0 | 0.030 ± 0.000 | 0.037 ± 0.009 | NS |
| C22:0 | 0.063 ± 0.005 | 0.040 ± 0.029 | NS | C22:0 | 0.043 ± 0.005 | 0.053 ± 0.019 | NS |
| C22:1 | 0.087 ± 0.005 | 0.053 ± 0.039 | NS | C22:1 | 0.050 ± 0.000 | 0.060 ± 0.014 | NS |
| C23:0 | 0.030 ± 0.000 | 0.023 ± 0.017 | NS | C23:0 | 0.020 ± 0.000 | 0.027 ± 0.009 | NS |
| C24:0 | 0.037 ± 0.005 | 0.027 ± 0.019 | NS | C24:0 | 0.023 ± 0.005 | 0.033 ± 0.012 | NS |
- TFA, trans fatty acid.
- NSP > 0.05 (non-significant).
Animal diet is also a known confounder, both in isolation and through interactions that occur between specific diets and specific genetics (Vance et al. 2012). Diet has not been controlled within all studies and is certainly not controlled between studies, adding further complexity. Within this study, animal diet was controlled, and under these controlled conditions, no significant differences were observed in fatty acid profiles. Comparing this study to investigations where diet was not controlled, it should be considered that conclusions made about fatty acid profiles in response to crossbreeding might actually be attributable to dietary differences rather than to breed.
As with the milk investigated in this study, no significant differences in fatty acid profile were observed between HF and JFX cheese (Table 4) (P > 0.05). The previous discussion in relation to milk-related nutrition, public health and TFA profiles can therefore also be considered within the context of cheese, this aspect of milk quality and lack of differences also being transferred to, and represented within, the Cheddar manufactured from the milk.
Cheese free fatty acid profile
Differences in FFAs can be used as an indicator of dairy product quality, and are an important flavour component in Cheddar cheese. With reference to Table 5, no significant differences in FFA levels were observed at 180 days between the JFX and HF treatment groups (P > 0.05). Overall, for both treatments, the FFA results observed in this study are within a comparable range of typical results for full-fat Cheddar cheese (Kilcawley et al. 2007).
| HF | JFX | P-value | |
|---|---|---|---|
| C4 | 169 ± 105 | 129 ± 36 | NS |
| C6 | 70 ± 62 | 57 ± 28 | NS |
| C8 | 73 ± 58 | 64 ± 27 | NS |
| C10 | 168 ± 111 | 151 ± 51 | NS |
| C12 | 164 ± 91 | 146 ± 42 | NS |
| C14 | 392 ± 190 | 346 ± 107 | NS |
| C16 | 928 ± 403 | 803 ± 224 | NS |
| C18 | 300 ± 152 | 254 ± 64 | NS |
| C18:1 | 882 ± 638 | 634 ± 304 | NS |
| C18:2 | 61 ± 35 | 55 ± 21 | NS |
| C18:3 | 54 ± 34 | 48 ± 17 | NS |
- NSP > 0.05 (non-significant).
Volatile-organic-compounds
Volatile organic compound levels can impact the sensory properties of cheese with both positive and negative results for consumer enjoyment of the food. The volatile compounds identified within all cheese consisted of six acids, eight alcohols, five aldehydes, three alkanes, one alkene, eight benzenes, one diol, seven esters, one ether, eight ketones, two pyrazines and one sulphur compound (Table S1). In total, 51 volatile compounds were identified, but amongst all of these compounds, no significant differences were observed in response to the crossbreeding factor (P > 0.05). The variances were noticeably high, although high variances are not atypical within this type of analysis, and similar ranges of values have been obtained previously within Cheddar research (O'Callaghan et al. 2017; Page et al. 2024). Overall, these results might be explained through trial-related impacts on volatile profile, which appear to be more consequential to this attribute within this study, as opposed to any differences related to the crossbreeding variable under consideration.
CONCLUSIONS
In terms of protein and fat content, the compositions of HF and JFX milk were significantly different from each other, but the compositions of HF and JFX Cheddar did not differ significantly. Manufacturing Cheddar with JFX milk resulted in an average ~5 min faster set-to-cut time and actual yield increase of ~3%; these differences were not statistically significant but could warrant future investigation. Indeed, all results from this study could be further validated at commercial scale; this study was completed at pilot scale. No meaningful differences were found in TFA profile, texture, proteolysis, pH, volatile organic compounds, and FFA profile between HF and JFX Cheddar. Overall, it is not evident that this crossbreeding strategy results in negative effects on the quality and nutritional attributes of Cheddar. Indeed, the significantly increased yellow colour and likely increased β-carotene content should align favourably with nutritional and quality preferences. However, to acknowledge some limitations of this work, when pondering consumer choices, the price paid by the consumer to buy the product also needs to be considered, and an economic analysis of the relative costs required to manufacture JFX Cheddar as compared to HF Cheddar did not form any part of this study. Likewise, sensory panel studies were not performed within these investigations, and any consideration of possible consumer choices is acknowledged as being limited to conjecture based on the interpretation of results from laboratory analyses, backed up by a reading of the literature. Crossbreeding is broadly accepted as a way of improving genetic diversity, herd health and fertility. This research suggests that a commonly practised crossbreeding strategy can be applied without adverse impacts on milk and Cheddar quality. However, the lack of significant impacts also suggests attempts to improve cheese quality through crossbreeding might not work; when targeting certain production phenotypes, dairy breeding strategies might need to focus on individual genetic markers for production traits as opposed to breed categories. Crossbreeding is already commonplace in New Zealand, and it could become more readily applied in other international locations. Overall, this research suggests that the quality of Cheddar cheese is not negatively impacted when manufactured with JFX milk, a valuable suggestion for all who might consider using this milk within dairy processing.
ACKNOWLEDGEMENTS
This work was funded through Teagasc internal funding, Project: Futuremilk2025 (grant number: 1412). Richard Page is a recipient of the Teagasc Walsh Scholarship. The authors would like to thank the Teagasc Animal and Grassland Research and Innovation Centre for the management of the breeding system and for providing milk for this work, particularly the contribution of Alann Jezequel. The authors also thank Chr. Hansen Ltd. for the donation of starter cultures and chymosin. Thank you for the support of colleagues in The Teagasc Food Chemistry and Technology Department, particularly Jimmy Flynn, Vijaya Lakshmi Chirumamilla, Sheila Cogan and Iwona Skibinska.
AUTHOR CONTRIBUTIONS
Richard M Page: Conceptualization; methodology; data curation; investigation; validation; formal analysis; visualization; project administration; resources; writing – original draft; writing – review and editing. John T Tobin: Validation; funding acquisition; resources; conceptualization. Kieran N Kilcawley: Methodology; investigation; validation; writing – review and editing. David T Mannion: Methodology; investigation; validation; writing – review and editing. Brendan Horan: Conceptualization; methodology; validation; resources; writing – review and editing; project administration. James A O'Mahony: Conceptualization; supervision. Tom F O'Callaghan: Conceptualization; methodology; validation; supervision; resources; project administration; writing – review and editing; funding acquisition. Prabin Lamichhane: Conceptualization; methodology; validation; investigation; data curation; resources; writing – review and editing; supervision; project administration; funding acquisition.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




