MBL2 deficiency is associated with higher genomic bacterial loads during meningococcemia in young children
Abstract
Mannose binding lectin (MBL2) is a soluble pattern recognition receptor that is key to generating innate immune responses to invasive infection, including against the cardinal Gram-negative bacterium Neisseria meningitidis. Individuals homozygous or heterozygous for any of three variant alleles of MBL2 (O/O or A/O genotypes) have deficient concentrations of MBL2 in circulating blood, but previous studies linking MBL deficiency to susceptibility to meningococcal disease have not revealed a consistent association. We genotyped 741 patients with microbiologically – proven meningococcal disease and correlated MBL2 genotype with plasma bacterial load of N. meningitidis with blood samples taken during hospital admission. We show that individuals with genotypes compatible with MBL2 deficiency have higher measurable levels of bacterial plasma genomic load with the greatest effect seen in children <2 years of age. However, the overall impact of this is minor, because there was no evidence that such genotypes are more common in children with meningococcal disease compared with uninfected cohorts. The findings suggest that MBL2 supports innate immune defence against meningococcal disease in the early months of life, before acquired immunity is sufficiently robust for effective natural protection.
Background
Infections caused by Neisseria meningitidis (N. meningitidis) remain an important cause of morbidity and mortality, despite the implementation of glycoconjugate vaccination in some parts of the world. Protection against meningococcaemia relies on the bactericidal activity of blood through activation of the complement cascade. In adults, this primarily occurs via specific immunoglobulin (the classical pathway), although in early life, innate mechanisms are critically important. The most common innate defect currently known is mannose-binding lectin (MBL2) deficiency, which affects c. 5–30% of adults 1. Although studies of the relationship between MBL2 deficiency and susceptibility to meningococcal infection have yielded conflicting information 2-5, there is evidence that low levels of this soluble pattern recognition molecule are associated with worse outcomes during sepsis and septic shock 6. For example, a multi-centre study of 88 children by Faber and colleagues, suggested that MBL exon 1 structural variants are more common in meningococcal disease patients compared to the general population, particularly in those aged <24 months 4.
In humans, MBL2 is encoded by 4 exons on the long arm of chromosome 10; polymorphism of exon 1 or of several highly pleomorphic promoter regions results in seven common haplotypes of MBL2 and variable plasma concentrations of MBL2 protein. Individuals not possessing the wild-type gene ‘A’ but who are instead homozygous or heterozygous for any of the three variant alleles (‘B’, rs1800450; ‘C’, rs1800451rs; ‘D’, rs5030737; or collectively ‘O’) are unable to form high order oligomers. The O/O or A/O genotypes result in plasma MBL2 concentrations c. 1% and 10% of A/A individuals, respectively 7. A recent meta-analysis estimated O allele frequency as 0.23 in the white European general population 5. Additional effects of promoter region variation is less well understood, although Y variants of the −221 G/C (rs7096206) base pair X/Y polymorphism tend to have higher levels of circulating MBL2 protein 8.
In the UK, rollout of the serogroup C glycoconjugate vaccination programme commenced November 1999. In the current study, we used a large, well-characterised, pre-conjugate vaccine cohort to determine whether MBL2 deficiency is associated with meningococcal infection acquisition in young children, and also whether there may be an association with measured plasma bacterial load or meningococcal disease severity/outcome.
Methods
Patients, samples and quantitative meningococcal PCR
The national Meningococcal Reference Unit for England and Wales (MRU) offers confirmatory testing on samples submitted from cases of suspected meningococcal infection. Testing includes both routine microbiological culture in addition to non-culture based diagnosis using EDTA-treated blood samples, as described previously 9. Patient and sample data were included in this analysis if an EDTA-treated blood sample had been submitted to the MRU and the patient was subsequently proven to have meningococcal disease on the basis of culture or PCR of material from an invasive site, and informed consent had been obtained. The Multiplex polymerase chain reaction (PCR)-based assay for detecting specific DNA sequences, including the N. meningitidis-specific capsule transport gene (ctrA), provides accurate data regarding genomic bacterial load in the range log10 3–9 copies/mL whole blood 10. Ethics committee approval for the study was granted by the Public Health Laboratory Service (now Public Health England) and the Ethics Committee of the Sheffield Teaching Hospitals NHS Foundation Trust (Sheffield, England).
MBL2 genotyping
Where residual DNA was available after processing of EDTA blood received in the period January 1999 through to December 1999 for quantitative meningococcal PCR, MBL2 structural genotypes were determined by heteroduplex and linkage disequilibrium analyses, as previously described 8, 11. This cohort was unlikely to be exposed to glycoconjugate vaccine. To determine allele frequency in a contemporary healthy population, a subset of the 14 000 children participating in the Avon Longitudinal Study of Parents and Children (ALSPAC) who had been genotyped for known structural and promoter polymorphisms of the MBL2 gene were used as a normal, unselected control group (see http://www.bristol.ac.uk/alspac/).
Statistical analysis
Based on previous suggestions that risk of N. meningitidis infection maybe increased in individuals possessing the defective MBL2 allele and our findings of poor outcomes from infection being related to N. meningitidis bacterial load and infecting serogroup, we hypothesized:
- that we may be able to identify an increased proportion of susceptible individuals in our national cohort compared to a contemporary control data set (the Avon cohort) and,
- that an age-related effect of MBL genotype on bacterial load may have an impact on the frequency of adverse outcomes.
Given the differences found in circulating levels of MBL associated with various allele combinations, we assumed that the most likely biologically plausible difference in outcome would be between O/O homozygous and the ‘wild-type’ (A/−) populations. Following preliminary testing by univariate analyses (using chi squared or Student's T test, as appropriate), individuals with alleles resulting in structurally variant MBL2 (i.e. B, C and D) were grouped together as ‘O’ therefore, in order to identify whether MBL2 deficiency contributed to mortality or measured plasma bacterial load using multivariate regression modelling. Bacterial load data were log10 transformed to assume a normal distribution. Statistical analysis was performed using SPSS software (version 20; IBM) and Prism (version 6; GraphPad, Inc.). Interactions were considered significant at p <0.05.
Results
Patients
MBL2 genotyping was conducted on 741 samples, and collated with clinical and microbiological data. The baseline demographic and bacteriologic characteristics of this meningococcal cohort were similar to those described previously relating to 1910 EDTA-treated blood samples from patients with proven meningococcal disease, collected January 1999 – December 2001 9 which included the current samples analysed here. The median age [IQR] was 5 [1-17] years, 50.9% were male gender and the meningococcal infection mortality rate was 8.6%. Infections were due mostly to serogroup B (45.9%) and C (36.3%) N. meningitidis, as was typical of this pre-vaccine era. The demographic, clinical and microbiological data derived from the total cohort of meningococcal disease patients, and the subset genotyped at MBL2 are each summarised in Table 1.
| ALL | Genotyped at MBL2 | |
|---|---|---|
| Number of patients | 1910 | 741 |
| Male gender, % | 50.5a | 50.9b |
| Mortality, n (%) | 170 (8.9)c | 63 (8.6) |
| Age, median years [IQR] | 5 [1–18] | 5 [1–17] |
| Infecting Neisseria meningitidis serogroup, n (%) | ||
| A | 1 (0.1) | 1 (0.1) |
| B | 1020 (54.1) | 334 (45.9) |
| C | 623 (33) | 264 (36.3) |
| Y | 8 (0.4) | 0 (0) |
| W135 | 31 (1.6) | 4 (0.6) |
| Ungrouped | 203 (10.8) | 124 (17.1) |
| Method of diagnosis | ||
| PCR only | 984 (51.5) | 336 (45.3) |
| Culture only | 251 (13.1) | 127 (17.1) |
| Both PCR & culture | 661 (34.6) | 271 (36.6) |
| Pre-hospital antibiotics received, n (%) | 275 (31.4) | 138 (34) |
| Time to PCR sample submission, median days (range) | 0 (0–25) | 0 (0–11) |
| Proportion PCR samples submitted within 24 h of hospital admission | 57.5 | 60.4% |
| Ethnicity, n (%) | ||
| White | 937 (96.9) | 414 (96.7) |
| Pakistani | 12 (1.2) | 4 (0.19) |
| Mixed | 12 (1.2) | 8 (1.9) |
| Other | 6 (0.6) | 2 (0.4) |
- IQR, interquartile range; PCR, polymerase chain reaction.
- a Information not available: 16/1910.
- b Information not available: 4/741.
- c Information not available: 21/1910.
Allele frequency & susceptibility to meningococcal infection
The frequency of O/O genotype was 0.072 (53/741) in patients with confirmed meningococcal infection, in comparison to 0.056 (59/1046) in individuals from the healthy control population (p NS). Excluding infected patients of non-Caucasian self-reported ethnicity resulted in an allele frequency of 0.065.
The frequency of A/O carriage in patients was 0.332 compared to 0.322 in the healthy control population. As expected, carriage of the B variant minor allele was most common (0.15) whereas the C and D alleles were less commonly found (0.02 and 0.07 respectively).
Observed allele frequencies did not differ by age group and no increased risk of meningococcal infection was seen in O/O genotype children <1 year of age (9/98 meningococcal patients vs. 8/104 healthy controls, p NS). The age-specific frequencies of SNPs found the current population in comparison to the cohort recently described by Bradley and colleagues 5 are detailed in Table 2.
| Age-group | WTCC controls (from Bradley and colleagues 5) (n = 5196) | |||||
|---|---|---|---|---|---|---|
| ALL (n = 741) | Aged < 1 (n = 109) | Aged 1–2 (n = 87) | Aged < 2 (n = 196) | Aged ≥ 2 (n = 545) | ||
| Genotype n, (frequency) | ||||||
| AA | 442 (0.6) | 63 (0.58) | 52 (0.6) | 115 (0.59) | 327 (0.6) | 3080 (0.59) |
| AO | 246 (0.33) | 38 (0.35) | 30 (0.34) | 68 (0.35) | 178 (0.33) | 1826 (0.35) |
| AB | 153 (0.21) | 22 (0.2) | 19 (0.22) | 41 (0.21) | 112 (0.21) | 1128 (0.22) |
| AC | 24 (0.03) | 5 (0.05) | 5 (0.06) | 10 (0.05) | 14 (0.03) | 154 (0.03) |
| AD | 69 (0.07) | 11 (0.10) | 6 (0.07) | 17 (0.09) | 52 (0.10) | 544 (0.10) |
| OO | 53 (0.07) | 8 (0.07) | 5 (0.06) | 13 (0.07) | 40 (0.07) | 290 (0.06) |
| BB | 23 (0.03) | 3 (0.03) | 1 (0.01) | 4 (0.02) | 19 (0.04) | 111 (0.02) |
| BC | 4 (0.005) | 1 (0.01) | 2 (0.02) | 3 (0.03) | 1 (0.002) | 29 (0.01) |
| BD | 14 (0.02) | 2 (0.02) | 0 | 2 (0.01) | 12 (0.02) | 107 (0.02) |
| CC | 3 (0.004) | 1 (0.01) | 0 | 1 (0.005) | 2 (0.004) | 2 (0.00) |
| CD | 3 (0.004) | 0 | 1 (0.01) | 1 (0.005) | 2 (0.004) | 13 (0.00) |
| DD | 6 (0.008) | 1 (0.01) | 1 (0.01) | 2 (0.01) | 4 (0.07) | 29 (0.01) |
| Simplified genotype | ||||||
| A | 1130 (0.76) | 164 (0.75) | 134 (0.77) | 136 (0.60) | 832 (0.76) | 7986 (0.77) |
| O | 352 (0.24) | 54 (0.25) | 40 (0.23) | 94 (0.41) | 258 (0.24) | 2406 (0.23) |
| B | 217 (0.15) | 31 (0.14) | 23 (0.13) | 54 (0.23) | 163 (0.15) | 1485 (0.14) |
| C | 37 (0.02) | 8 (0.04) | 8 (0.05) | 16 (0.07) | 21 (0.02) | 199 (0.02) |
| D | 98 (0.07) | 15 (0.07) | 9 (0.05) | 24 (0.10) | 74 (0.07) | 722 (0.07) |
Survival after meningococcal infection
No significant difference was seen in the survival rate of patients homozygous for O alleles (O/O) compared to patients with A/O or A/A combinations (92.3% vs. 91.3% respectively; p NS, chi squared test). Survival rates remained similar when stratified by patient age group (data not shown).
Bacterial load during infection
Neisseria meningitidis quantification data were available for 307/741 patients for whom an EDTA-treated blood sample was received by the reference laboratory. The bacterial load measured tended to be higher in patients homozygous for O alleles (O/O, n = 21) than in ‘wild-type’ patients (A/−, n = 285) (median copy number [IQR], 4.72 [3.66–5.86] vs. 4.30 [3.53–5.38]; p 0.054, T-test).
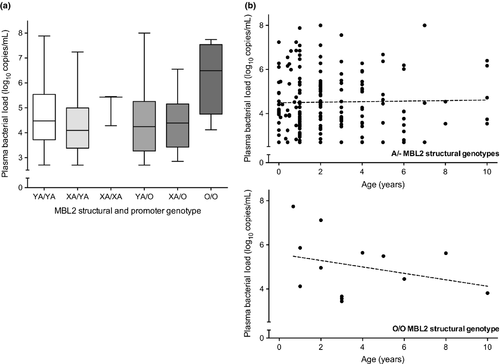
Patients <2 years of age with the O/O genotype (n = 4) had significantly higher bacterial loads than those patients with an A/− genotype (median [IQR], 6.67 [4.99–7.61] vs. 4.14 [3.47–5.33]; p 0.007, T-test), regardless of additional MBL2 promoter SNP polymorphisms (X/Y variants, see Fig. 1a). In general the bacterial load measured in O/O genotype patients appeared to diminish with age, whereas no similar decline was seen in the ‘wild-type’ population, but was non-significant (Pearson's correlation, p NS, see Fig. 1b).
Factors affecting bacterial load
To assess the combination of factors affecting the measured circulating bacterial load, we included O/O genotype homozygosity into our previously described multivariate model for bacterial load 9. Both serogroup C infection and possession of the MBL2 O/O genotype but not patient age, significantly contributed towards an increased plasma bacterial load; O/O genotype was associated with a 1.858 log10 higher (95% CI, 1.019–3.388, p 0.043) and serogroup C infection with a 1.414 log10 higher N. meningitidis plasma bacterial load (95% CI, 1.036–1.928, p 0.029), in a sample of 300 patients for whom data was available with all other factors being equal (Table 3).
| Effectb | 95% CI | p Value | |
|---|---|---|---|
| Homozygous for deficient MBL2 allele (OO)c | 1.858 | 1.019–3.388 | 0.043 |
| Serogroup C infection | 1.414 | 1.036–1.928 | 0.029 |
- CI, confidence interval.
- a Data for 300 patients for whom both the MBL2 structure and infecting serogroup was known.
- b Average measured increase in log10 plasma bacterial load for individuals homozygous for MBL2 deficient alleles or infected with serogroup C infection, all other factors being equal. Other variables included in the model were serogroup B infection and age.
- c To form the model a recessive effect of MBL2 was assumed to convey a biologically meaningful impact on circulating MBL in plasma.
Discussion
We show, for the first time, that individuals with genotypes compatible with MBL2 deficiency have higher measurable levels of bacterial plasma genomic load during a defined invasive infection. We found no evidence that such genotypes are more common overall in children with meningococcal infection, even in children <1 year of age, consistent with the large case–control study by Bradley and colleagues 5.
Although our sample did not reveal an effect of MBL2 genotype on susceptibility to meningococcal invasion and disease, our data suggests that control of early bacterial replication within blood may be impeded, as is expected with this defect of innate immunity. This implies a subtle contribution by MBL2 to innate immunity, that is insufficient to render individuals grossly susceptible to invasive disease. Previous in vitro data suggests that MBL phenotype together with circulating levels of IL1β and IL10 may have a combinatorial effect on the pro-inflammatory cytokine cascade 12, and that MBL2 promotes non-opsonic phagocytosis of N. meningitidis by human macrophages 13. Thus, in individuals homozygous for the defective MBL2 alleles, a reduced level of MBL2 and associated reduction in the levels of inflammation may give rise to a higher detected peripheral bacterial load. An additional contributing factor is likely to be the nature of the infecting serogroup; as replicated here, we have previously demonstrated that patients infected with serogroup C N. meningitidis have significantly higher plasma bacterial loads 9. Higher bacterial loads worsen disease severity, but we were not able to demonstrate conclusively a direct relationship between MBL2 genotype, bacterial genomic load, and mortality in this study, because of the small number of deaths in the sample. The effect of MBL2 deficiency on measured plasma bacterial loads was most marked in the first 2 years of life underscoring the important high-risk period of early infancy prior to the development of specific bactericidal anti-meningococcal antibodies. Interestingly, MBL2 variants associated with MBL deficiency also are more frequent in HIV-infected children with more rapid HIV-1 disease progression and CNS impairment, with the greatest effect observed in children younger than 2 years 14.
The strengths of this study include the relatively large sample and both the validation of meningococcal disease and measurement of bacterial genomic load by a national reference laboratory. Weaknesses include the relative inaccuracy of history-obtained ethnicity data; in the era of whole genome sequencing the ability to define ancestry by genetic clustering is the gold standard. This study was also a retrospective analysis of previously collated data, although the allele frequency and disease incidence rates are in keeping with other large multi-centre studies.
Conclusions
In conclusion, in children with genotypes associated with deficiency of MBL2 there is an age-related inverse correlation with the number of bacteria present in the circulation, highlighting the importance of developing protective humoral response mechanisms and underscoring the requirement of an effective vaccine to protect this at-risk age group. These data support the assertion that MBL2 deficiency per se does not grossly increase the risk of acquiring meningococcal infection, but appears to be involved in controlling bacterial numbers in younger children once acquisition has occurred. In view of the association between plasma bacterial load and death 9, it is likely that larger studies would show a relationship between MBL2 genotype and mortality in Gram-negative infection.
Funding
This work was supported by the Meningitis Research Foundation [award number 4/00].
Acknowledgements
We thank the Consultants in Communicable Disease Control, the patients, healthy control participants and their families for assistance in the construction of the original database.
Transparency Declaration
All authors declare no conflicts of interest.