Development of a reporter gene assay for antibody dependent cellular cytotoxicity activity determination of anti-rabies virus glycoprotein antibodies
Wenbo Wang and Chuanfei Yu contributed equally to this work
Abstract
Rabies is a viral disease that is nearly 100% fatal once clinical signs and symptoms develop. Post-exposure prophylaxis can efficiently prevent rabies, and antibody (Ab) induction by vaccination or passive immunization of human rabies immunoglobulin (HRIG) or monoclonal antibodies (mAbs) play an integral role in prevention against rabies. In addition to their capacity to neutralize viruses, antibodies exert their antiviral effects by antibody-dependent cellular cytotoxicity (ADCC), which plays an important role in antiviral immunity and clearance of viral infections. For antibodies against rabies virus (RABV), evaluation of ADCC activity was neglected. Here, we developed a robust cell-based reporter gene assay (RGA) for the determination of the ADCC activity of anti-RABV antibodies using CVS-N2c-293 cells, which stably express the glycoprotein (G) of RABV strain CVS-N2c as target cells, and Jurkat cells, which stably express FcγRⅢa and nuclear factor of activated T cells (NFAT) reporter gene as effector cells (Jurkat/NFAT-luc/FcγRⅢa cells). The experimental parameters were carefully optimized, and the established ADCC assay was systematically validated according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q2 guideline. We also evaluated the ADCC activity of anti-RABV antibodies, including mAbs, HRIG, and vaccine induced antisera, and found that all test antibodies exhibited ADCC activity with varied strengths. The established RGA provides a novel method for evaluating the ADCC of anti-RABV antibodies.
Abbreviations
-
- Ab
-
- antibody
-
- ADCC
-
- antibody-dependent cellular cytotoxicity
-
- ADCP
-
- antibody-dependent cellular phagocytosis
-
- BBB
-
- blood brain barrier
-
- bsAb
-
- bispecific antibody
-
- CDC
-
- complement-dependent cytotoxicity
-
- CNS
-
- central nervous system
-
- EBOV
-
- Ebola virus
-
- EC50
-
- half-maximal effective concentration
-
- Fab
-
- fragment antigen binding
-
- FACS
-
- fluorescence-activated cell sorting
-
- Fc
-
- fragment crystallizable
-
- G
-
- glycoprotein
-
- HIV
-
- human immunodeficiency virus
-
- HRIG
-
- human rabies immunoglobulin
-
- ICH
-
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
-
- ID50
-
- half-maximal inhibitory dilution
-
- mAb
-
- monoclonal antibody
-
- NFAT
-
- nuclear factor of activated T cells
-
- NK cell
-
- natural killer cell
-
- NS
-
- national standard
-
- NIFDC
-
- National Institutes for Food and Drug Control
-
- OIE
-
- World Organization for Animal Health
-
- PBMC
-
- peripheral blood mononuclear cells
-
- PEP
-
- post exposure prophylaxis
-
- PMA
-
- phorbol 12-myristate 13-acetate
-
- RABV
-
- rabies virus
-
- RGA
-
- reporter gene assay
-
- RIG
-
- rabies immunoglobulin
-
- RLU
-
- relative luciferase units
-
- SEC
-
- size exclusion chromatography
-
- S/N
-
- signal to noise ratio
-
- TCID50
-
- tissue culture infectious dose
-
- WHO
-
- World Health Organization
-
- ZIKV
-
- Zika virus
INTRODUCTION
Fatal rabies can be prevented by timely and correct administration of post exposure prophylaxis (PEP), which consists of vaccination and passive immunization of rabies immunoglobulins (RIGs). The neutralizing activity of antibodies against rabies virus (RABV) is critical to ensuring against RABV infection,1 and according to the World Health Organization (WHO) and World Organization for Animal Health (OIE), serum titers of neutralizing antibodies ≥0.5 IU/mL are considered adequate for rabies protection. In addition to neutralization activity, Fc effector functions of antiviral antibodies such as antibody-dependent cellular cytotoxicity (ADCC) are important attributes in the clearance of virus-infected cells, and benefits have been effectively demonstrated in human immunodeficiency virus (HIV), Ebola virus (EBOV), Zika virus (ZIKV), influenza virus, and SARS-CoV-2.2-9 However, information on the contributions of ADCC in PEP is limited, and evaluation of such functions of rabies biologics for PEP has been neglected.
ADCC is initiated by the interaction of the fragment antigen-binding (Fab) and fragment crystallizable (Fc) region of antibodies with virus and Fcγ receptors on effector cells such as natural killer (NK) cells, macrophages, eosinophils, and neutrophils. This subsequently induces effector cells to release cytotoxic substances such as perforin, resulting in viral elimination via infected cell lysis and inhibition of viral spread. The traditional ADCC assay that uses peripheral blood mononuclear cells or NK cells isolated from human blood as effector cells is not suitable for routine quantitative measurement of ADCC activity due to poor reproducibility and low sensitivity. Our laboratory has established several reporter gene-based bioactivity determination methods,10-13 including the ADCC assay for anti-MERS antibodies,14 and these methods have obvious advantages compared with traditional methods. For RABV, to date, only a few studies have demonstrated that anti-RABV antibodies and lymphocytes from human peripheral blood could mediate the lysis of infected cells by ADCC.15, 16
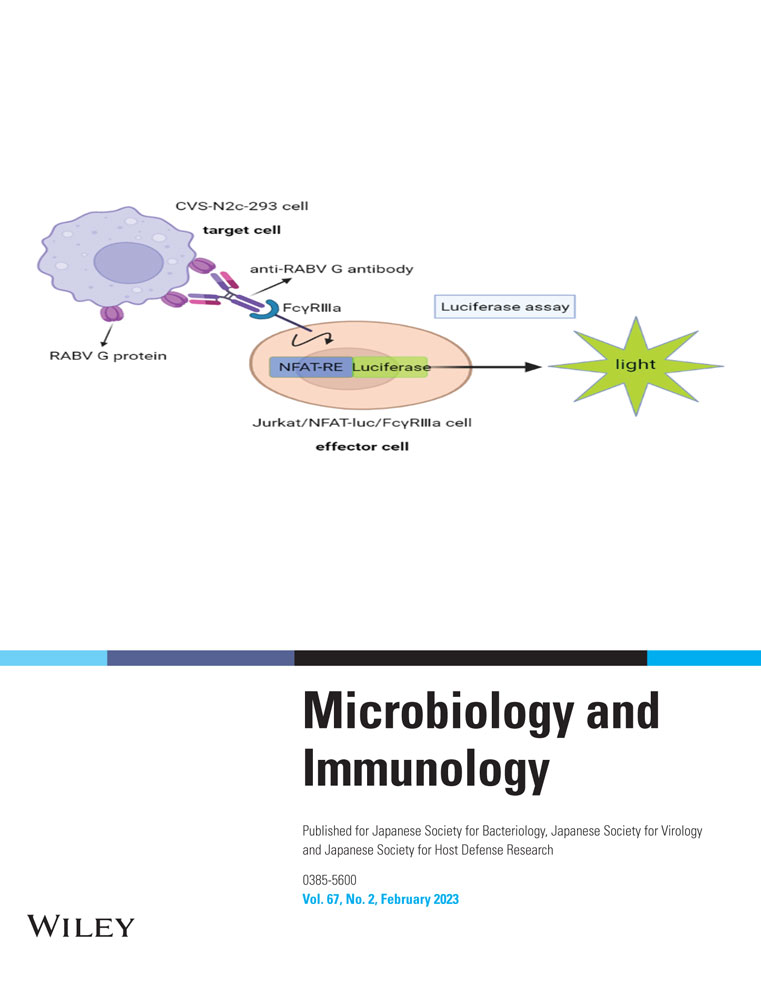
In this study, we developed a cell-based reporter gene assay (RGA) for measuring the ADCC activity of anti-RABV-G antibodies using target CVS-N2c-293 cells that stably express the RABV G protein on their surface, effector cells Jurkat/NFAT-luc/FcγRⅢa that stably express FcγRⅢa, and luciferase reporter genes driven by NFAT response elements (Figure 1a). This ADCC assay showed good performance characteristics, including high specificity, accuracy, and precision. Using this assay, the ADCC activity of anti-RABV antibodies, including HRIG, vaccine-induced antisera, and mAbs were evaluated.
MATERIALS AND METHODS
Jurkat/NFAT-luc/FcγRⅢa as effector cells for the ADCC RGA assay
The ADCC effector cells Jurkat/NFAT-luc/FcγRⅢa were developed as described previously.14 Briefly, Jurkat T cells were first transfected by electroporation with plasmid pcDNA3.1, which expresses the human FcγRⅢa, and G418-resistant cell clones were screened by fluorescence-activated cell sorting for FcγRⅢa expression. The selected cell clone was then transfected with plasmid pcDNA3.1-puro that encodes the luciferase reporter under the control of the NFAT promoter, and puromycin-resistant cell clones were screened by phorbol 12-myristate 13-acetate (PMA) and ionomycin co-stimulation for high induction of relative luciferase units (RLU). The dual-resistant Jurkat/NFAT-luc/FcγRⅢa cell clone was confirmed by determining the ADCC activity of the anti-HER2 antibody against SK-BR-3 cells.
Generation of the CVS-N2c-293 cell line as target cells
The HEK293 cells were transfected with plasmid pcDNA3.1 that encodes the CVS-N2c G protein17 and cultured in selective medium (complete medium with 500 µg/mL G418). G418-resistant single cell clones were obtained by limited dilutions, then screened for clones that highly expressed luciferase and low background by co-culturing with Jurkat/NFAT-luc/FcγRⅢa effector cells and gradient dilutions of anti-RABV mAb-1. mAb-1 is a human IgG1 antibody that targets the antigenic site III of RABV G. The expression of the RABV G protein in the selected cell clone was determined by flow cytometry and named as the CVS-N2c-293 cell line.
ADCC RGA
CVS-N2c-293 cells were seeded into white-bottom 96-well plates at a density of 30,000 cells per well and incubated at 37°C and 5% CO2 for 16–20 hr. After incubation, the supernatant in each well was removed, and 50 μL of three-fold serially diluted anti-RABV G mAb (20–0.019 µg/mL) in dilution buffer (RPMI-1640, 0.5% BSA) was added into the plate. Then, 240,000 cells per well of Jurkat/NFAT-luc/FcγRⅢa cells14 were added and incubated at 37°C in a humidified atmosphere with 5% CO2. After 6 hr of incubation, 100 μL Bright-Glo luciferase assay reagent (Promega) was added into each well, and RLUs were recorded with a plate reader (Envision, PerkinElmer).
Anti-RABV G mAbs and vaccine-induced antisera
Seven anti-RABV neutralizing mAbs targeting different epitopes were included, and mAb-1 was used for method optimization. Both mAb-1 and mAb-2 target antigenic site III, mAb-3 binds to the discontinuous conserved residues, mAb-4 targets antigenic site I, whereas mAb-5 targets antigenic site II. Bispecific antibody (bsAb), bsAb-1, and -2 target antigenic sites I and II and their Fc regions were mutationally optimized to enhance binding affinity to FcγRⅢa. mAb-2 and -3 are components of an anti-RABV cocktail that are in a phase III clinical trial, and mAb-4 has been approved for clinical use in China.
Four anti-RABV sera, namely YRS, DN, TX, and PD, were obtained by immunization of vaccine comprising the Flury-LEP strain. A commercial HRIG was obtained from Tonrol Bio-Pharmaceutical Co., Ltd (Hefei, China). The 37.0 IU/mL national standard (NS) for anti-RIG was established and calibrated by the National Institutes for Food and Drug Control (NIFDC).
Pseudovirus-based neutralization assays
Pseudotyped RABV preparation, titration, and neutralization assays were performed as described previously.17 The pseudovirus-based neutralization assay was measured as a reduction in luciferase expression after a single-round infection of 293 T cells. Briefly, 100 μL serial diluted antisera or mAbs was added into 96-well plates, and 50 μL pseudoviruses (32,000 tissue culture infectious dose [TCID50]/mL) were added, then incubated at 37°C for 1 hr. After incubation, 100 μL of 293 T cells (4 × 105 cells/mL) was added in the presence of 10 μg/mL DEAE-dextran in a total volume of 250 μL, then incubated at 37°C in a humidified atmosphere with 5% CO2. The luciferase activity was measured after 48 hr of incubation.
Method validation of established ADCC RGA assay
According to the ICH Q2 (R1) guideline, the specificity, accuracy, precision, and linearity of the ADCC assay were systematically validated. Anti-RABV G mAb-1 was diluted to seven concentrations of 5, 10, 15, 20, 25, 30, and 35 μg/mL, and another prepared sample (20 μg/mL) was used as the reference. The nominal relative bioactivity of anti-RABV mAb-1 dilutions should be 25%, 50%, 75%, 100%, 125%, 150%, and 175%, respectively. The ratio of measured relative bioactivity versus the nominal value indicated the accuracy of the RGA, and the linearity was demonstrated by plotting the measured versus the expected relative bioactivity. Repeatability and intermediate precision were assessed by testing the 100% level samples (20 μg/mL) thrice on three different days by three operators independently.
Data analysis
A four-parameter model was used to fit the dose–response curve between antibody concentrations (log10) and RLU by Prism software 5.0. The relative ADCC potency was calculated by dividing the half-maximal effective concentration (EC50) of the reference by EC50 of the tested sample. The Reed–Muench method was used to calculate the half-maximal inhibitory dilution (ID50) of the antisera.
RESULTS
Generation of the CVS-N2c-293 cell line as target cell for the ADCC assay
The CVS-N2c-293 cell line that stably expresses the G protein of the CVS-N2c strain was generated by transfecting HEK293 cells with plasmid pcDNA3.1 that encodes the CVS-N2c G protein, followed by selection with G418. Limited dilutions were applied to obtain monoclonal cell clones. Finally, 10 clones were obtained and isolated for effective induction of luciferase expression and a full dose–response curve using Jurkat/NFAT-luc/FcγRⅢa as the effector cell for the ADCC assay. Clone 197, which showed the highest induced luminescence intensity and signal-to-noise ratio (S/N) value (Figure 1b), was selected as the target cell clone for further method optimization. The expression of the G protein of the CVS-N2c strain on the cell surface of clone 197 was also confirmed by flow cytometry analysis (Figure 1c).
Optimization of the ADCC RGA
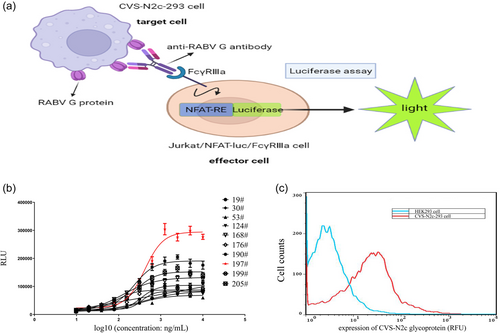
Key parameters of the ADCC assay, including the density of the target cells, the ratio of effector cells to target cells (E:T ratio), range of mAb working concentration, and induction time, were further optimized. The density of the target cells (CVS-N2c-293 cell) was adjusted to 0.5 × 104, 1 × 104, 3 × 104, and 5 × 104 cells/well (Figure 2a–d), and the density of effector cells (Jurkat/NFAT-luc/FcγRⅢa cell) was correspondingly adjusted at E:T ratios of 1:1, 2:1, 4:1, and 8:1. The dose–response curve was well fitted, and a high S/N was obtained when the density of CVS-N2c-293 cell was 3 × 104 cells/well and the E:T ratio was 8:1 (Figure 2c and Table 1). Then, the anti-RABV mAb was pre-diluted to 20 μg/mL (working concentration: 10 μg/mL), and serial dilutions consisting of 10 concentration points with different dilution folds (2-, 3-, 4-, or 5-fold) were prepared. The optimal range of working concentration was the three fold serial dilution, and the concentration range was 0.152–10,000 ng/mL (Figure 2e). The induction time was optimized by co-culturing the target and effector cells for 2 hr, 4 hr, 6 hr, 8 hr, 10 hr, 12 hr, 22 hr, and 24 hr, and we observed the highest S/N at 6 hr (Figure 2f). Taken together, key parameters were optimized to obtain a well-fitted dose–response curve with a high S/N to determine the ADCC activity of anti-RABV G antibodies.
| Target cell density | E:T ratios | R2 | S/N |
|---|---|---|---|
| 5,000 cells per well | 1:1 | 0.021 | 1.2 |
| 2:1 | 0.010 | 1.0 | |
| 4:1 | 0.390 | 2.2 | |
| 8:1 | 0.868 | 2.8 | |
| 10,000 cells per well | 1:1 | 0.527 | 2.7 |
| 2:1 | 0.634 | 3.0 | |
| 4:1 | 0.817 | 12.2 | |
| 8:1 | 0.995 | 16.6 | |
| 30,000 cells per well | 1:1 | 0.944 | 14.4 |
| 2:1 | 0.967 | 12.3 | |
| 4:1 | 0.982 | 16.4 | |
| 8:1 | 0.997 | 18.1 | |
| 50,000 cells per well | 1:1 | 0.947 | 25.9 |
| 2:1 | 0.989 | 25.1 | |
| 4:1 | 0.992 | 20.2 | |
| 8:1 | 0.984 | 8.3 |
Validation of the ADCC RGA
According to ICH Q2 (R1) guideline, the specificity, accuracy, linearity, and precision of the ADCC assay were validated. We found mAbs that target non-RABV epitopes, including the trastuzumab (anti-HER2 mAb), pembrolizumab (anti-PD-1 mAb), bevacizumab (anti-VEGF mAb), infliximab (anti-TNFα mAb), rituximab (anti-CD20 mAb), and denosumab (anti-RANKL mAb) could not induce luciferase expression or generate a dose–response curve in our established ADCC assay, where only the anti-RABV G mAb showed a specific signal curve (Figure 2a). These data indicated that our established ADCC assay is highly specific (Figure 3).
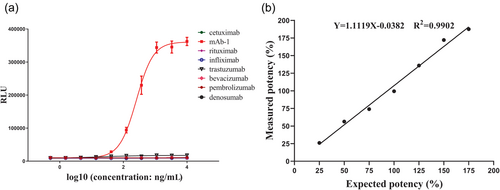
mAb-1 was pre-diluted to seven samples at different concentrations with an expected potency of 25%, 50%, 75%, 100%, 125%, 150%, and 175%. The ADCC activity of all seven samples were measured, and the relative bioactivities were calculated using another independent 100% level sample as the reference. The recoveries of the seven samples with different bioactivity levels were within 100 ± 15% (Table 2), which indicated good accuracy. Linearity was demonstrated by plotting the measured versus the expected relative bioactivity with a fitted R2 > 0.99 (Figure 2b).
| Expected potency (%) | 25 | 50 | 75 | 100 | 125 | 150 | 175 |
| Measured potency (%) | 26.03 | 56.38 | 73.96 | 99.48 | 135.95 | 172.20 | 187.60 |
| Recovery (%) | 104.12 | 112.76 | 98.61 | 99.48 | 108.76 | 114.80 | 107.20 |
The repeatability and intermediate precision of the RGA assay were assessed by testing the 100% level samples thrice on three different days by three operators (O1, O2, and O3) independently. The RSD of relative bioactivities measured from plate to plate, day to day, and operator to operator was below 9%, which indicated good repeatability (plate-to-plate precision) and intermediate precision (day-to-day and operator-to-operator precision) (Table 3).
| Operator | Day | Relative potency (%) | Plate to plate (%) | Day to day (%) | Operator to operator (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plate1 | Plate2 | Plate3 | ± S | RSD | ± S | RSD | ± S | RSD | ||
| O1 | D1 | 108.37 | 104.72 | 101.68 | 104.93 ± 3.35 | 3.19 | 102.46 ± 5.94 | 5.80 | ||
| D2 | 98.92 | 107.98 | 113.40 | 106.77 ± 7.32 | 6.85 | |||||
| D3 | 96.04 | 100.33 | 90.68 | 95.68 ± 4.83 | 5.05 | |||||
| O2 | D1 | 105.28 | 107.29 | 94.92 | 102.49 ± 6.64 | 6.48 | 102.52 ± 1.35 | 1.32 | 102.46 ± 0.06 | 0.06 |
| D2 | 96.07 | 104.93 | 102.56 | 101.19 ± 4.59 | 4.54 | |||||
| D3 | 101.92 | 109.61 | 100.14 | 103.89 ± 5.03 | 4.84 | |||||
| O3 | D1 | 99.48 | 97.93 | 108.26 | 101.89 ± 5.57 | 5.47 | 102.41 ± 4.23 | 4.13 | ||
| D2 | 111.43 | 96.89 | 112.32 | 106.88 ± 8.66 | 8.11 | |||||
| D3 | 88.65 | 102.70 | 104.04 | 98.46 ± 8.53 | 8.66 | |||||
Further applications of the RGA
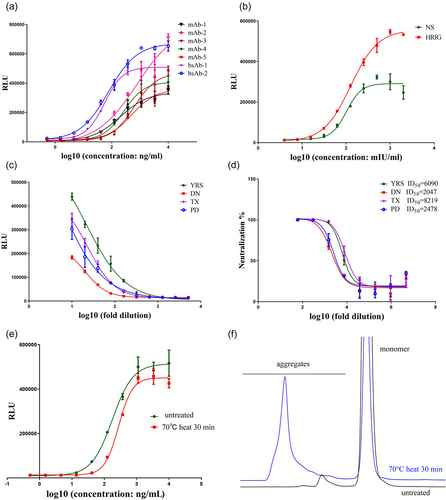
Using the established RGA, we measured the ADCC activity of anti-RABV Abs (five mAbs and two bsAb) and also the anti-RABV polyclonal antibodies, including one HRIG, one NS, and four vaccine-induced antisera. Seven anti-RABV Abs targeting different antigenic sites of the RABV glycoprotein showed dose–response curves but different ADCC strengths (Figure 4a), which indicated that this assay can be used in assessing the relative ADCC bioactivities of anti-RABV Abs. Polyclonal antibodies HRIG and NS were pre-diluted to 16 IU/mL (resulting in an initial working concentration of 8 IU/mL) and prepared in two-fold serial dilutions to generate 10 concentration points. As shown in Figure 4b, both HRIG and NS showed dose–response curves and comparable ADCC potencies (EC50: 101.8 mIU/mL for NS, 127.5 mIU/mL for HRIG). It is of note that the HRIG induced much higher RLU compared with NS. The vaccine-induced antisera also showed ADCC activity (Figure 4c), and we found that there was no correlation between ADCC strength and neutralization potency (Figure 4d). Although sera DN had the lowest neutralization potency and also showed the lowest ADCC activity (lowest RLU value), the other three sera varied. The neutralizing Ab titer of sera TX was three-fold higher than sera PD; however, the ADCC strength of these two sera was similar (comparable RLU values). Sera YRS, which had an intermediate-level neutralizing Ab titer, showed the most potent ADCC activity. We also found that the ADCC activity of mAb-1, which was denatured by heating at 70°C for 30 min, had decreased compared with the untreated mAb-1 (Figure 4e), whereas a higher percentage of aggregates was also detected in heated mAb-1 (47.9% vs. 2.6%, Figure 4f), suggesting that the established ADCC assay may be utilized in assessing the stability of anti-RABV mAbs.
DISCUSSION
RABV is a neurotropic virus that, upon entry into the body, replicates in muscle or other local tissues and gains access to motor endplates and motor axons to reach the central nervous system (CNS). The incubation period of rabies ranges from 5 days to many years (usually 2–3 months; rarely >1 year). It is commonly considered that once RABV enters the “immunologically privileged” CNS, antiviral antibodies could not be efficiently transported across the blood–brain barrier (BBB), and the antiviral functions such as ADCC hardly work. Indeed, BBB provides a physiological separation of the CNS from the periphery and thus cells and molecules cannot easily enter the CNS,18-20 leading to challenges in treatment against infections and cancers in the CNS. However, through an effective delivery system or molecular engineering, antibodies could efficiently be transported into the CNS, and numerous studies have proven that sufficient delivery of anti-RABV antibodies into the brain via the BBB with permeability enhancement is crucial in the clearance of RABV from the brain and improve the survival rate of infected mice, thereby providing an effective treatment of CNS infection.21-25 Additionally, the “immunologically privileged” CNS has macrophages,26 microglia,27-29 and astroglial cells,30 all of which harbor Fc receptors and could act as effector cells for ADCC. Microglial and astroglial cells can adopt an activated state with upregulation of FcγRs, which clear invading pathogens or lyse human tumor cells by triggering ADCC.28, 30
Although research in ADCC against RABV infection is limited, evidence has prompted us to hypothesize that ADCC may play roles against RABV infection in both the CNS and the periphery. Studies have shown that vaccine-induced antibodies, and/or therapeutic mAbs could induce ADCC against CNS cancers both in vitro and in vivo.31-34 In particular, anti-GD2 antibodies with major anti-neuroblastoma mechanisms of ADCC,35-37 could improve the cure rate of metastatic CNS neuroblastoma38 and have been applied effectively to high-risk neuroblastoma. For RABV, it has been reported that the protective activity of a particular RABV mAb in vivo did not correlate with its virus-neutralizing activity in vitro,39 indicating that Fc effector functions may contribute to protection effectiveness. A recent study has reported that administration of an RABV mAb cocktail (RVC20 and RVC58) in the periphery and CNS through intracerebroventricular infusion cures symptomatic rabid mice by improving their clinical condition, exhibiting undetectable viral loads, and almost normal inflammatory profile, whereas the same treatment of mAb cocktail with LALA mutation (L234A and L235A) failed to protect mice.40 The LALA mutation in Fc region of antibody retained the same neutralizing activity and in vivo pharmacokinetics, but abrogated binding to Fc-gamma receptors, suggesting that Fc effector functions may play an important role in the clearance of RABV from the CNS. Thus, it is expected that through appropriate delivery or administrate approaches, anti-RABV antibodies could mediate ADCC to lyse RABV infected cells in the CNS and the periphery.
In the present study, we established a cell-based RGA for measuring the ADCC activity of anti-RABV-G antibodies through key parameter optimization and validated this assay according to the ICH_Q2_R1 guideline. The established RGA for ADCC is robust and reliable and was applied to evaluate the ADCC activity of HRIG, vaccine-induced antisera, and mAbs targeting different epitopes. We found that all the tested HRIG, antisera, and mAbs showed ADCC activity, although their strength varied. The tested RABV mAbs targeting different epitopes, including antigenic sites I, II, and III, mediated ADCC activity, and bsAb-1 and bsAb-2 with an Fc region that was optimized to enhance its affinity to FcγRⅢa, showed the most potent ADCC activity. Whether RABV mAbs target other epitopes such as antigenic site IV, G5, and minor a, and whether non-neutralizing antibodies have ADCC activity will be investigated further. We also found that the ADCC activity of the four tested antisera was not obviously correlated to the neutralization potency (Figure 4d). The non-neutralizing antibodies can also confer protection to viral infection by ADCC,41, 42 and for RABV, the functions and properties of vaccine-generated antibodies such as binding activity, neutralizing/non-neutralizing Ab ratio, ADCC activity, and their relationship need further investigation. These findings indicate that our assay can be used in assessing the ADCC bioactivities of anti-RABV antibodies, including the HRIG, vaccine-induced antisera, and mAbs, and could also be utilized in assessing mAb stability.
Due to the global shortage and high price of HRIG, WHO recommended the use of mAbs as alternatives for RIG in PEP, and to date, several mAbs have been approved or are in clinical trials.43-45 As an integral part of PEP where vaccination usually takes time to induce an immune response, passive immunization of anti-RABV mAbs could neutralize RABV and lyse infected cells by ADCC immediately after RABV exposure. Another advantage of mAbs is that these could be optimized and engineered to a specific purpose; for example, ADCC activity could be enhanced by optimal mutations of amino acids in the Fc region or optimization of Fc glycosylation by glycoengineering.46, 47 In addition, mAbs could be re-engineered as bispecific antibodies or fusion proteins that additionally target endogenous BBB transporters to enhance their transport efficiency into the CNS.48-50 Thus, an engineered anti-RABV mAb with enhanced ADCC activity and CNS transport efficiency maybe more efficient against RABV infection in both the CNS and the periphery.
Apart from ADCC, other Fc effector functions such as antibody-dependent cellular phagocytosis (ADCP) and complement-dependent cytotoxicity (CDC) play roles in antiviral infection. We evaluated the ADCP and CDC activity of anti-RABV antibodies using CVS-N2c-293 cells as target cells and found that several anti-RABV antibodies exhibit both ADCP and CDC activity with varied strengths (data not shown). The two methods we established will be systematically validated and more anti-RABV antibodies, including mAbs, HRIG, and vaccine-induced antisera, will be further investigated by our laboratory.
CONCLUSION
We developed a robust reporter gene-based ADCC assay to determine the bioactivity of anti-RABV-G antibodies. This assay could be used in evaluating the ADCC activity of vaccine-induced sera, HRIG, and mAbs, and should be a useful tool in the characterization and lot release of anti-RABV antibodies. Although the tested anti-RABV antibodies in the current study showed ADCC activity in our established assay, further studies involving live animals will be necessary to investigate clearly the contribution of ADCC in anti-RABV infection.
AUTHOR CONTRIBUTIONS
Wenbo Wang, Youchun Wang, and Lan Wang conceived and designed the experiments. Wenbo Wang and Chuanfei Yu performed the experiments and analyzed data. Chunyu Liu, Yalan Yang, Gangling Xu, Gang Wu, Jialiang Du, Zhihao Fu, Luyong Guo, Caifeng Long, and Xijie Xia performed the method validation. YC performed the size exclusion chromatography. Wenbo Wang and Youchun Wang wrote the paper. Yuhua Li provided the national standard (NS) for anti-RIG. All authors read and approved the final document.
ACKNOWLEDGMENTS
The authors thank all who provided mAbs, HRIG, and antisera for these studies. This work was supported by the National Key Research and Development Program of China [grant number 2021YFF0600804].
DISCLOSURE STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.