Changes of frequency and expression level of CD161 in CD8+ T cells and natural killer T cells in peripheral blood of patients with systemic lupus erythematosus
Abstract
Immunologic abnormalities of natural killer (NK) cells and T cells play a role in the pathogenesis of systemic lupus erythematosus (SLE). CD161 is expressed on most of the NK cells and on some T cells. The quantities of CD161-expressing cells and expression levels of CD161 were analyzed in T cells and NK cells from patients with SLE compared with normal controls. The expression of CD161 on NK cells, NKT cells, CD4+ T cells, and CD8+ T cells in peripheral blood from patients with inactive SLE and active SLE, and from the normal controls group were determined using flow cytometry. The frequency and expression level of CD161 in the lymphocyte subsets and its relationship with the quantity of regulatory T cells, anti-double stranded DNA antibody, and the titer of antinuclear antibody were evaluated. Both the percentages of the CD161+ subpopulation and the mean fluorescence intensities (MFIs) of CD161 in CD8+ T cells and NKT cells decreased significantly in SLE patients compared with normal controls (P < .001). The CD161 expression in CD8+ T cells and NKT cells also decreased in the anti-dsDNA (+) group (P < .05). The counts of Treg cells were lower in SLE patients and were weakly correlated with the percentage of the CD161 subpopulation (r = 0.229, P = .016) and the MFIs of CD161 expression in CD8+ T cells (r = .232, P = .014). The frequencies and levels of CD161 expression on CD8+ T cells and NKT cells were reduced in SLE patients, suggesting that an abnormality of these cells was related to the pathogenesis of SLE.
Abbreviations
-
- anti-dsDNA
-
- anti-double stranded DNA antibody
-
- CLEC5B
-
- C-type lectin domain family 5 member B
-
- FoxP3
-
- Forkhead box protein P3
-
- FS
-
- forward scatter
-
- KLRB1
-
- killer cell lectin-like receptor subfamily B member 1
-
- mAb
-
- monoclonal antibody
-
- MFIs
-
- mean fluorescence intensities
-
- NK
-
- natural killer
-
- NKR-P1A
-
- NK cell receptor protein 1A
-
- PBMCs
-
- peripheral blood mononuclear cells
-
- PBS
-
- phosphate-buffered saline
-
- SLE
-
- systemic lupus erythematosus
-
- SLEDAI
-
- SLE disease activity index
-
- SS
-
- side scatter
-
- Th17
-
- T helper 17
-
- Treg
-
- Regulatory T
1 INTRODUCTION
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease affecting multiple organs. Although the pathogenesis of SLE remains unclear, this disease is characterized by autoantibody production resulting from immune dysregulation and the breakdown of self-tolerance.1 Regulatory T (Treg) cells, a subset of CD4+ T cells, maintain self-tolerance by preventing the activation/proliferation of autoreactive T cells. The number and function of Treg cells have been studied in SLE patients and quantitative and qualitative defects of Treg cells were observed in some studies,2, 3 but other studies found no defects.4, 5 Besides Treg cells, natural killer (NK) cells play a role in the development of SLE, because NK cells are able to inhibit autoreactive T helper 17 (Th17) cells.6 Several studies reported decreased NK cell numbers and cytotoxicity function, impaired NK cell differentiation, and altered cytokine production in SLE patients.7-9
Some surface molecules share common expression between leukocyte subsets. One of these molecules is CD161 expressed on the majority of NK cells and on some T cells. CD161, which is also referred to as NK cell receptor protein 1A (NKR-P1A), killer cell lectin-like receptor subfamily B member 1 (KLRB1), or C-type lectin domain family 5 member B (CLEC5B), is a type II transmembrane C-type lectin glycoprotein.10 Some studies suggested that CD161 was a marker of pro-inflammatory NK cell function and might be associated with T cells of memory phenotype.11, 12 Although the role of CD161 molecules in SLE pathogenesis has not been explored comprehensively, a few studies showed that SLE patients had selective defects in the quantities of CD161-expressing NK cells, and SLE flares might be linked to the expansion of Th17 cells with the depletion of Treg cells.13, 14 In addition, the frequency and the absolute number of CD161+CD8+ cells decrease in patients with rheumatic diseases, including SLE.15 Numerical and functional deficiencies of NKT cells were found in SLE patients, suggesting that NKT cells played a suppressive role in the pathogenesis of SLE.16 The frequency of CD161 expressing cells and the intensity of CD161 in CD3-CD56+ NK cells and CD3+CD56+ NKT cells were reduced in SLE patients compared with those in normal controls.14
The present study evaluated the frequencies and levels of CD161 expression in the peripheral blood NK cells, NKT cells, CD4+ T cells, and CD8+ T cells from patients with active SLE and inactive SLE, and a normal controls group. In addition, we investigated whether the quantity of Treg, the presence of anti-double stranded DNA antibody (anti-dsDNA), and the titer of antinuclear antibody (ANA) were related to any abnormality of the CD161 expression.
2 MATERIALS AND METHODS
2.1 Study population
The study population comprised 61 SLE patients and 50 normal controls. We retrospectively reviewed the clinical data for each patient. The demographic characteristics of the normal controls, patients with active SLE, inactive SLE, and the total SLE group are described in Table 1. SLE was diagnosed using the classification criteria of the American College of Rheumatology.17 The disease activity of SLE was determined using the SLE disease activity index (SLEDAI) scoring method, and a score of ≥ 4 defined the active disease.18 All SLE patients had been receiving hydroxychloroquine (200 mg or 300 mg daily). Glucocorticoids and immunosuppressive drugs were administered in 53 and 20 patients, respectively. This study was approved by the Institutional Review Board for Research Ethics of our institution.
| Active SLE (n = 31) | Inactive SLE (n = 30) | Total SLE (n = 61) | Normal controls (n = 50) | |
|---|---|---|---|---|
| Age (median [range]) | 36 (22–83) | 43 (19–77) | 38 (19–83) | 42 (23–65) |
| Sex (n [%]) | ||||
| Male | 5 (16.1) | 3 (10.0) | 8 (13.1) | 10 (20.0) |
| Female | 26 (83.9) | 27 (90.0) | 53 (86.9) | 40 (80.0) |
| SELDAI score | ||||
| Median (range) | 7 (4–12) | 2 (0–3) | 4 (0–12) | – |
| ANA titer (n [%]) | ||||
| 1:80–1:320 | 14 (45.2) | 18 (60.0) | 32 (52.5) | – |
| ≥1:640 | 17 (54.8) | 12 (40.0) | 29 (47.5) | – |
| Anti-dsDNA Abs | ||||
| Positive (n [%]) | 28 (90.3) | 10 (33.3) | 38 (62.3) | – |
| Median (range) | 188 (0–621) | 0 (0–193) | 58 (0–621) | – |
- ANA, antinuclear antibody; SLE, systemic lupus erythematosus; SLEDAI, systemic lupus erythematosus disease activity index.
2.2 Flow cytometric analysis
Collected heparinized whole blood was diluted 1:2 with phosphate-buffered saline (PBS). For purifying peripheral blood mononuclear cells (PBMCs) from whole blood, Ficoll-Paque density gradient centrifugation was used within 4 hr after blood collection. To analyze NK and T cell subsets, the following monoclonal antibody (mAb) to human antigens were used: anti-CD3 APC Alexa Fluor 750 (Beckman Coulter, Fullerton, CA), anti-CD4 pacific blue (PB; Beckman Coulter), anti-CD8 phycoerythrin-cyanine 7 (PC7; Beckman Coulter), anti-CD56 APC (Beckman Coulter), anti-CD161 fluorescein-isothiocyanate (FITC; BD Biosciences, San Jose, CA), and anti-RORγ (Retinoid-related orphan receptor gamma) phycoerythrin (PE; BD Biosciences). The PBMC (1 × 106 cells) were stained with 10 μL of anti-CD3-APC Alexa Fluor 750 mAb, 10 μL of anti-CD4-PB mAb, 10 μL of anti-CD8-PC7 mAb, 10 μL of anti-CD56-APC mAb, 20 μL of anti-CD161-FITC mAb, and 5 μL of anti-RORγ-PE mAb. The mixture was incubated for 40 min at room temperature (20–25°C) in the dark and washed three times with PBS prior to analysis. Isotype-matched antibody controls were used to detect nonspecific staining. Flow cytometric analysis was performed using a Navios flow cytometer (Beckman Coulter). At first, the lymphocytes were gated in a forward scatter (FS) and a side scatter (SS), and, then, the lymphocyte population was classified into CD3-positive and CD3-negative lymphocyte populations (Figure S1). The CD3-positive lymphocyte population was differentiated into CD4+CD8−CD56−, CD4−CD8+CD56−, and CD4−CD8−CD56+ lymphocytes for the subsequent analysis of the expression pattern of CD161. In the CD3−CD56+ lymphocyte population, the expression patterns of CD161 were also analyzed. The percentages and mean fluorescence intensities (MFIs) of the CD161+ fraction in CD4+ T cell, CD8+ T cell, NKT cell, and NK cell subsets were also examined. For CD4+ T cells, CD161+ RORγ cells were defined as Th17 cells, and the percentage of Th17 cells was measured. To analyze Treg cells, the PBMC (1 × 106 cells) were incubated with 10 μL of anti-CD4-FITC mAb, 10 μL of anti-CD25-PE mAb, and 10 μL of anti-CD127-PC5 mAb. These mAbs were purchased from Beckman Coulter. The CD4+ T cells were gated from lymphocytes. The fraction of CD25+CD127− cells on gated CD4+ T cells was analyzed.
2.3 Statistical analysis
All statistical analyses were performed using MedCalc statistical software 14.12.0 (MedCalc Software, Mariakerke, Belgium). The Kolmogorov-Smirnov test was used to assess normality of the variables. The data did not follow a normal distribution. Therefore, the data are presented as the median (interquartile range), unless otherwise stated. In the intergroup comparisons for non-parametric data, the Mann-Whitney rank sum test or the Kruskal-Wallis test followed by Dunn's multiple comparison test were used. To assess the correlations between two parameters, the linear regressions were calculated. A value of P less than .05 was considered significant.
3 RESULTS
In analysis of the lymphocyte subsets, the percentage of Treg cells in SLE patients (4.97 [4.16–6.09]) showed a lower value than that in normal controls (5.47 [4.73–6.70]; P = .015). The count of Treg cells was significantly lower in patients with SLE [0.02 (0.01–0.03)] than that in normal controls (0.04 [0.03–0.05] × 103/μL; P < .001), and it was lower in patients with active SLE (0.01 × 103/μL) compared with those with inactive SLE (0.03 × 103/μL, P = .029). The percentage of Th17 cells (CD4+CD161+RORγ+) showed no significant difference between the SLE patients and the normal controls (P = .545).
In patients with active SLE, the expression of CD161 was detected in 12.1% (8.22–15.1) of CD4+ T cells, 9.10% (6.46–14.9) of CD8+ T cells, 60.1% (37.7–71.0) of NK cells, and 28.3% (22.3–52.7) of NKT cells (Figure 1). In the patients with inactive SLE, the expression of CD161 was observed in 10.9% (8.07–18.2) of CD4+ T cells, 14.5% (9.24–18.8) of CD8+ T cells, 65.3% (62.4–76.6) of NK cells, and 48.4% (26.8–55.7) of NKT cells. For the normal controls, CD161 was expressed in 13.2% (11.2–20.9) of CD4+ T cells, 22.7% (15.2–31.3) of CD8+ T cells, 63.2% (50.2–75.5) of NK cells, and 53.3% (47.2–64.0) of NKT cells. The frequencies of CD161+CD8+ cells and CD161+CD3+CD56+ cells were significantly lower in both active and inactive SLE patients than those in normal controls. The frequencies of CD161+CD4+ cells and CD161+CD3−CD56+ cells were not different in either active or inactive SLE patients compared with that of the normal controls.
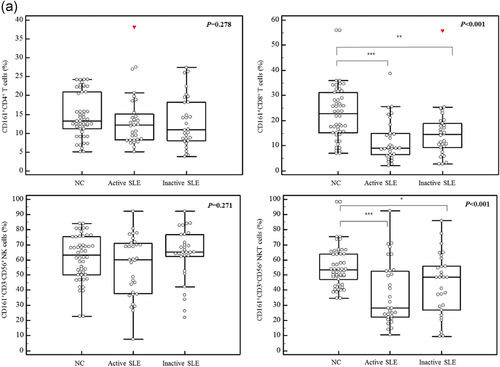
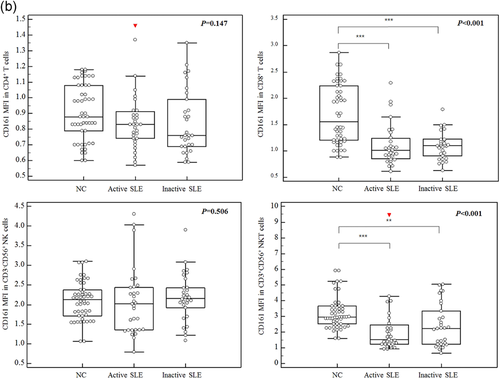
The MFIs of CD161 were 0.83 (0.74–0.91) in CD4+ cells, 1.01 (0.85–1.24) in CD8+ T cells, 2.02 (1.36–2.45) in CD3−CD56+ cells, and 1.52 (1.21–2.47) in CD3+CD56+ cells in active SLE patients (Figure 1). The MFIs of CD161+ cells of each lymphocyte subset were not statistically different between the active and inactive SLE patients. However, compared with normal controls, active and inactive SLE patients showed reduced MFIs of CD161 in CD8+ T cells and CD3+CD56+ NKT cells, but not in CD4+ T cells and CD3−CD56+ NK cells.
Among the 61 SLE patients, 29 patients had a high titer (≥1:640) of ANA. The frequencies of CD161+ cells in CD4+ T cells (P = .829), CD8+ T cells (P = .583), NK cells (P = .954), and NKT cells (P = .740) showed no significant difference between patients with high ANA titers and patients with mild to moderate ANA titers (1:80–1:320). The MFIs of CD161 in CD4+ T cells (P = .593), CD8+ T cells (P = .729), NK cells (P = .812), and NKT cells (P = .588) were not different between patients with high ANA titers and patients with mild to moderate ANA titers (1:80–1:320).
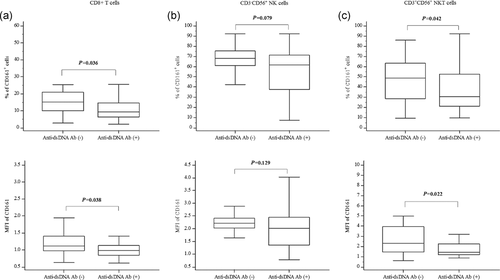
The anti-dsDNA Abs were positive in 38 patients. The presence of anti-dsDNA Abs was not affected by the frequency of CD161+ cells in CD4+ T cells (P = .557) and CD3-CD56+ NK cells (P = .079). However, the frequencies of CD161+ cells in CD8+ T (9.32% vs. 15.2%, P = .036) and NKT cells (30.7% vs. 48.6%, P = .042) were lower in the anti-dsDNA-positive group compared with the anti-dsDNA-negative group (Figure 2). Moreover, the MFIs in CD8+ cells (0.99 vs. 1.12, P = .038) and in CD3+CD56+ cells (1.42 vs. 2.31, P = .022) were significantly lower in the anti-dsDNA-positive group than those in the anti-dsDNA-negative group. However, the MFIs of CD161 in CD4+ cells (P = .639) and CD3−CD56+ cells (P = .129) showed no difference between the anti-dsDNA-positive and negative groups.
The high titer of ANA and the presence of anti-dsDNA did not affect the percentage (P = .608 and P = .480, respectively) and count (P = .093 and P = .273, respectively) of Th17 cells. There was a weak correlation between the count of lymphocytes and the frequency of the CD161+ subpopulation (r = 0.291, P = .002) or CD161 MFI (r = 0.320, P = .001) for CD8+ T cells but not for NKT cells (r = 0.129, P = .117 vs. r = 0.118, P = .218, respectively). The count of Treg cells was weakly correlated with the frequencies of the CD161+ subpopulation (r = 0.229, P = .016) and CD161 MFIs (r = 0.232, P = .014) in CD8+ T cells. However, the count of Treg cells was not related to the frequencies of the CD161+ subpopulation and CD161 MFI in NKT cells (r = 0.087, P = .362 vs. r = 0.039, P = .686, respectively).
4 DISCUSSION
CD161 is known to be expressed in the majority of NK cells and 24% of T cells, including NKT cells.19 In a previous study, SLE patients showed decreased frequencies of CD161+ cells NK cells and NKT cell populations and decreased levels of CD161 expression in CD161+ subpopulation of T cells and NKT cells compared with normal controls.14 This study indicated that SLE patients displayed a reduction in CD161 expression in CD8+ T cells and NKT cells, but the defect in CD161 expression in CD4+ T cells and NK cells was not proven. However, similar to the previous study,14 the expression level of CD161 was reduced in T cells and NKT cells. In particular, when the CD3+ T cells were divided into CD3+CD4+ T cells and CD3+CD8+ T cells, and in CD3+CD8+ T cells, the expression level of CD161 was decreased, but in CD3+CD4+ T cells, there was no change. Moreover, unlike the previous study,14 which included only SLE patients with a SELDAI score of more than 3, in the present study when the active SLE and inactive SLE groups, according to the SELDAI score, were divided, both groups showed a significant decrease in the frequency and expression level of CD161 in CD8+ T cells and NKT cells compared with the normal control group.
CD161 marks T cells with the ability to secrete interleukin-17 (IL-17), and CD161+ T cells can develop into Th17 cells and T cytotoxic 17 (Tc17) cells.11, 20 Active SLE patients presented higher proportions of IL-17 producers among the CD4+ cells and CD8+ cells compared with controls.21 In this study, the proportion of Th17 showed no significant difference between SLE patients and normal controls, and disease activity was not related to the proportion of Th17. On the other hand, the frequency of CD161+CD8+ T cells and the CD161 intensity in CD8+ T cells decreased in SLE. We speculate that CD161+CD8+ T cells may reflect not the population of Tc17 cells but the population having regulatory effects on autoimmunity. These findings show that the defect in CD161 expression in CD8+ T cells can contribute to the occurrence of SLE, but it is not evident whether SLE patients have a defect in CD161 expression in CD4+ T cells.
NKT cells are a subset of T cells that possess properties of both T cells and NK cells and play a suppressive role in SLE.16 Differentiated type I NKT cells (iNKT cells) exhibit an up-regulation of CD161 expression. Because iNKT cells can secrete Th2-biased cytokines, the reduced number of iNKT cells can cause an imbalance between Th1 and Th2 cytokines.22 The reduced number of iNKT cells is restored to the normal level after treatment and is correlated with the progression of disease in the lupus murine model.23, 24 In this study, we observed a reduction in the CD161+ subpopulation and CD161 intensity in NKT cells. These data could be related to the reduction in iNKT cells.
The reduction in CD161 expression in CD8+ T cells and NKT cells was associated with the presence of anti-dsDNA Abs in SLE patients. The degree of disease activity was not correlated with CD161 expression in these subsets. The frequency of positivity of anti-dsDNA Abs is higher during lupus flares, and the titers of Abs are higher in patients with active SLE compared with those with inactive SLE.25 Although the relation between CD161 expression in CD8+ T cells or NKT cells and disease activity was not proven because of the variable duration of treatment (median 38 months, range 3–154) and the relatively limited number of patients, positivity of anti-dsDNA Abs was accompanied by most active SLE patients. The Treg cell count was related to CD161 expression in CD8+ T cells. In a recent study,26 the Treg cells decreased in SLE patients and was correlated with the SLEDAI. Thus, we hypothesized that the reduction in CD161 expression in CD8+ T cells and NKT cells appeared in active SLE patients.
CD161 seems to function as an inhibitory receptor in NK cells. A previous study reported that SLE patients had a reduction in the percentage of CD161-expressing cells among the NK cells, but the expression level of CD161 was not affected.14 In our study, down-regulation of CD161 expression in NK cells was not significant in the SLE patients. We suggest that the functional significance of CD161 in NK cells had no clear role in the pathogenesis of SLE.
There are some limitations in this study. First, the number of patients with inactive SLE (n = 30) and with active SLE (n = 31) were relatively smaller than the normal controls (n = 50). Second, Treg cells were defined as CD4+CD25+CD127− expressing cells without FoxP3 or CD45RA in this study. Forkhead box protein P3 (FoxP3) is a key transcriptional factor for the development and differentiation of natural Treg cells and CD45RA is a T cell differentiation/activation marker. By using FoxP3 and CD45RA as an expression marker, CD4+ T cells could functionally divide into three subpopulations: CD45RA+FoxP3low resting Treg cells, CD45RA−FoxP3hi activated Treg cells, and cytokine-secreting CD45RA−FoxP3low nonsuppressive T cells.27 However, due to the difficulty of fixation and permeabilization for intracellular staining of FoxP3, the cell surface marker CD127 (α-chain of IL-7 receptor) which has a negative correlation with FoxP3, has been used as a suitable marker for the isolation of Treg cells.28-30 According to another study, when FoxP3 is added to the analysis, the number of Treg cells defined by CD25+CD127-FoxP3+ could be reduced, due to a fraction of CD25+CD127−FoxP3− which might be increased in patients with autoimmune disease such as rheumatoid arthritis.31 Therefore, further studies with additional markers such as FoxP3 and CD45RA will be needed for more accurate and functional analysis for Treg cells.
In conclusion, decreased frequencies of the CD161+ subpopulation and a reduced intensity of CD161 in CD8+ T cells and NKT cells are present in SLE patients. The count of Treg cells is associated with the expression of CD161 in CD8+ T cells. Our data indicate that the defect in CD161 in CD8+ T cells and NKT cells may influence the development of SLE.
ACKNOWLEDGMENTS
This research was supported by research fund of Chungnam National University Hospital, 2017.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.