The immune characterization of interferon-β responses in tuberculosis patients
ABSTRACT
We aimed to assess the immunoregulatory effects of IFN-β in patients with tuberculous pleurisy. IFN-β, IFN-γ and IL-17 expression levels were detected, and correlations among these factors in different culture groups were analyzed. Pleural fluid mononuclear cells (PFMC) from tuberculous pleural effusions, but not peripheral blood mononuclear cells (PBMC) from healthy donors, spontaneously expressed IFN-β, IL-17 and IFN-γ. Moreover, exogenous IFN-β significantly inhibited the expression of IL-17 in PFMC. By contrast, IFN-β simultaneously enhanced the levels of IFN-γ. To further investigate the regulation of IL-17 and IFN-γ by endogenous IFN-β, an IFN-β neutralizing antibody was simultaneously added to bacillus Calmette-Guérin (BCG)-stimulated PFMC. IL-17 expression was significantly increased, but IFN-γ production was markedly decreased in the experimental group supplemented with the IFN-β neutralizing antibody. Simultaneously, IL-17 production was remarkably increased in the experimental group supplemented with the IFN-γ neutralizing antibody. Taken together, in our study, we first found that freshly isolated PFMC, but not PBMC from healthy donors, spontaneously expressed IFN-β, IL-17 and IFN-γ in vivo. Moreover, IFN-β suppressed IL-17 expression and increased IFN-γ production. Furthermore, both IFN-β and IFN-γ down-regulated IL-17 expression. These observations suggest that caution is required when basing anti-tuberculosis treatment on the inhibition of IFN-β signaling.
List of Abbreviations
-
- BCG
-
- bacillus Calmette-Guérin
-
- ELISPOT
-
- enzyme-linked immunospot assay
-
- GAPDH
-
- glyceraldehyde-3-phosphate dehydrogenase
-
- HBV
-
- hepatitis B virus
-
- HCV
-
- hepatitis C virus
-
- HIV
-
- human immunodeficiency virus
-
- M. tb
-
- Mycobacterium tuberculosis
-
- PBMC
-
- peripheral blood mononuclear cell
-
- PFMC
-
- pleural fluid mononuclear cell
-
- TB
-
- tuberculosis
Tuberculous (TB) pleurisy is caused by exposure to Mycobacterium tuberculosis (M. tb), whose autolysis products metabolize into pleural inflammation caused by pleural cavity hypersensitivity. Tuberculous pleural effusion is caused by delayed hypersensitivity to M. tb, and most patients can self-heal. Currently, thoracic puncture fluid is routinely used for the treatment of exudative tuberculous pleurisy, and TB patients with pleural effusion are good models for studying the local immune status of TB. Some studies have shown that M. tb functions similarly to other pathogens in that infection is caused by an important response to type I IFN activation 1-3. IFN-β, a glycoprotein secreted by leukocytes and fibroblasts, is classified as a type I IFN that exerts antiviral, antitumor and immunomodulatory effects 4. However, the role of IFN-β in immunization against M. tb remains unclear.
IFN-γ is a specific glycoprotein produced mainly by the antigen and mitogen to stimulate the activation of T cells and NK cells. IFN-γ plays an essential role in TB protective immunity by promoting the proliferation and differentiation of T cells, macrophage activation, phagocytosis, lysosomal fusion etc. 5. IFN-γ also participates in TB granuloma immune responses and exerts an anti-TB immune function 6. However, the percentage of TB cases involving local IFN-β-mediated IFN-γ production is still unclear.
IL-17, mainly secreted by Th17 cells, plays a very important role in bodily anti-infective and inflammatory responses and exerts immune effects by inducing the secretion of various cytokines, thus recruiting protective cells to the site of infection. High IL-17 expression could also induce inflammatory cell aggregation and promote cellular phenotypic changes, ultimately resulting in immunopathological damage 7-10. The role of IL-17 in TB infection is still unclear, and there are no reports of IFN-β regulating IL-17 expression during TB infection.
Therefore, we investigated the effects of IFN-β on IL-17 and IFN-γ expression in pleural effusions from TB infected-patients. This study aimed to provide theoretical guidance for deeply understanding local immune responses to TB, systemic tuberculosis diagnoses and immune intervention.
MATERIALS AND METHODS
Subjects
A total of 35 patients newly diagnosed with TB pleurisy at Sun Yat-Sen Memorial Hospital were enrolled in this study. All the participants underwent diagnostic thoracenteses to obtain pleural fluid or pleural biopsy tissue before the start of chemotherapy. Diagnosis of TB pleuritis was based on positive cultures for M. tb in the pleurisy samples, clinical and radiological features and good responses to anti-TB treatment. Participants included 17 men and 18 women, with a mean age of 55.6 years (range, 19–87 years). Patients with HIV, HBV or HCV infection, history of autoimmune diseases or any malignant tumor were excluded from the study. Pleurisy samples were subjected to routine biochemical analysis, including tests for total protein (TP), glucose (Glu), lactate dehydrogenase (LDH) and adenosine (ADA). Leukocyte counts in the TB pleurisy samples ranged from 0.5 × 109 to 16.7 × 109 cells/L, and the purity of the lymphocytes ranged from 37% to 100%. Twenty-one healthy donors (10 men and 11 women, mean age of 34 ± 10 years, range 22–53 years) were also recruited from Sun Yat-Sen Memorial Hospital. We carried out subsequent experiments by randomly selecting 10 specimens from the PFMC of 35 TB pleuritis patients from each group, which were given different stimulants. Specimens from healthy donors were used only as controls. Adequate informed consent was obtained from all individuals involved in this study. This study was approved by the Sun Yat-Sen University Cancer Center. The authenticity of this article has been validated, as key raw data were uploaded onto the Research Data Deposit public platform (www.researchdata.org.cn) under the approval RDD number RDDA2017000313.
Cell preparation
PBMC from healthy donors and PFMC from TB pleural effusions were isolated by density gradient centrifugation using Ficoll-Hypaque. The cells were collected and washed twice in Hank's balanced salt solution, and their viabilities were tested using Trypan blue dye. The cells were suspended at a final concentration of 2 × 106 cells/mL in RPMI-1640 medium supplemented with 50 µM 2-mercaptoethanol, 2 mM l-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% heat-inactivated FCS. To further separate CD14+ and CD14−cells from the PFMC, CD14+ cells were collected with anti-CD14 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), and they showed a mean purity of 97% as determined by flow cytometry. CD14−and CD14+ cells were collected after stimulation with BCG. Total cellular RNA was extracted and analyzed for IFN-β expression by RT-PCR.
Antigens and antibodies
The following mAbs were used for surface and intracellular staining: CD3-PE, CD4-PerCP, IFN-γ-APC, IL-17-FITC, anti-IFN-γ, and isotype-matched antibodies were purchased from BD Biosciences Pharmingen (Franklin Lakes, NJ, USA). Purified IFN-β and anti-IFN-β mAbs were obtained from PBL Biomedical Laboratories (Piscataway, NJ, USA). BCG was purchased from the Chengdu Institute of Biological Products (Chengdu, China). In all cases, stimuli were used under the following conditions: 5 µg/mL BCG, 50 ng/mL IFN-β, 5 µg/mL anti-IFN-β, 5 µg/mL anti-IFN-γ, 5 µg/mL isotype-matched control antibodies unless otherwise indicated.
RT-PCR
RNA was extracted from PFMC and PBMC using an RNAeasy kit (Qiagen, Valencia, CA, USA), and residual DNA was treated according to the manufacturer's instructions. Reverse transcription was carried out using the PrimeScript RT-PCR Kit (Takara, Dalian, China). RT-PCR assays were conducted on a DNA thermal cycler (Applied Biosystems 2720; Thermo Fisher Scientific, Waltham, MA, USA) under the following conditions: 95°C for 5 min, 25–30 cycles at 94°C for 30 s, 62°C for 30 s, and 72°C for 60 s, followed by 72°C for 5 min. The following primer pairs (Takara) were used: GAPDH forward, 5′-GCATGGCCTTCCGTGTCC-3′ and GAPDH reverse, 5′-GAGTGTGGCAGGGACTC-3′; IFN-β forward, 5′-GTCTCCT CCAAATTGCTCTC-3′ and IFN-β reverse, 5′-ACAGGAGCTTCTGACACTGA-3′; IL-17 forward, 5′-TTAGGCCACATGGTGGACAATCGG-3′ and IL-17 reverse, 5′-GGCCACATGGTGGACAATCGG-3′; IFN-γ forward, 5′-GGCTTTTCAGCTCT GCATCGT-3′ and IFN-γ reverse, 5′-TCCACACTCTTTTGGATGCTCTGGT-3′. The amplified products were loaded onto 2% agarose gels containing ethidium bromide, and the bands were observed under UV irradiation.
ELISA
Culture supernatants were collected and examined for the production of IFN-γ (BD Biosciences Pharmingen), IFN-β and IL-17 (PBL Biomedical Laboratories) by ELISA according to the manufacturer's instructions. Detection sensitivities of the ELISA kits were 7.8 pg/mL for IFN-β, 4.7 pg/mL for IFN-γ and 7.8 pg/mL for IL-17.
ELISPOT
IL-17 and IFN-γ production was detected by the ELISPOT assay. Briefly, single-cell suspensions were prepared and plated on 96-well plates (Millipore, Billerica, MA, USA) with an anti-IL-17 (or anti-IFN-γ) capture antibody at 4°C overnight. The cells were then washed and blocked at room temperature with RPMI-1640 medium containing 10% FBS for 2 hr. A total of 2 × 105 PFMC were added to the wells in triplicate and then incubated with BCG in the presence of IFN-β mAbs for 48 hr. The cells were then washed and incubated with a biotinylated anti-IL-17 (or anti-IFN-γ) detection antibody (2 µg/mL) at room temperature for 2 hr. The wells were washed and incubated with HRP for 1 hr, and 3-amino-9-ethyl-carbazole (AEC) substrate solutions were then added. Next, spot development was monitored, and the reaction was quenched with water. After drying, an ELISPOT reader (Cellular Technology Limited, Shaker Heights, Cleveland, OH, USA) was used to count the number of spots per well.
Cell surface and intracellular cytokine staining
Cell suspensions were treated with BCG, BCG plus anti-IFN-β, BCG plus anti-IFN-γ, or BCG plus anti-IFN-β and anti-IFN-γ at 5% CO2 and 37°C for 24 hr. Brefeldin A (BFA, 10 mg/mL; Sigma-Aldrich, St Louis, MO, USA) was added within 8 hr of incubation completion. The cells were then washed twice, resuspended in PBS, and incubated with anti-CD3 and anti-CD4 for surface staining in the dark at 4°C for 30 min. After surface staining, the cells were washed twice and fixed in 4% paraformaldehyde, followed by permeabilization and staining in PBS containing 0.1% saponin for intracellular IL-17 cytokine detection. Cells were collected on a BD FACSAria II flow cytometer (Becton Dickinson, San Jose, CA, USA) and analyzed with FlowJo software (TreeStar, San Carlos, CA, USA).
Statistical analysis
Data are presented as the mean ± SD. Comparisons between two groups were carried out using paired or unpaired Student's t-tests. Multiple comparisons were evaluated by one-way anova. P < 0.05 was considered significant.
RESULTS
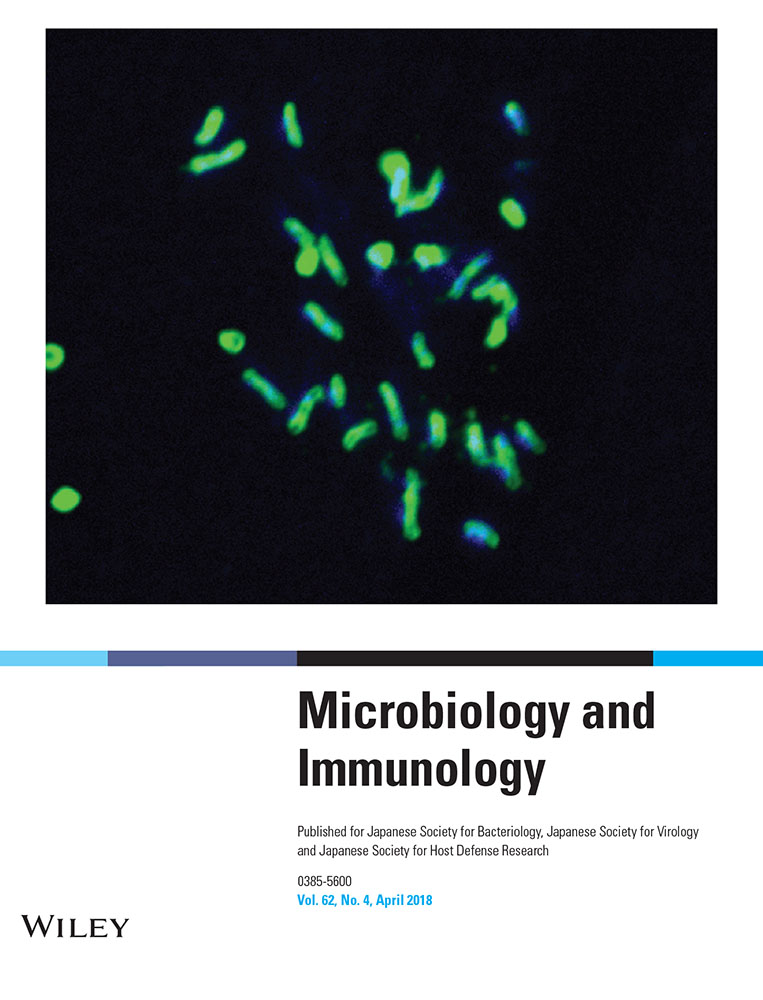
PFMC from tuberculous pleural effusion samples spontaneously expressed IFN-β
To investigate the contribution of IFN-β to M. tb infection, we examined its expression in PBMC freshly isolated from healthy individuals and PFMC from TB pleural effusions. Total RNA was extracted, and IFN-β expression was detected by RT-PCR. PFMC from tuberculous pleural effusion samples, but not PBMC from healthy individuals, spontaneously expressed IFN-β (Fig. 1a,b). Moreover, IFN-β expression was significantly increased in PFMC after stimulation with BCG in a time-dependent method (Fig. 1c,d). In addition, we used ELISA to detect IFN-β protein expression in pleural fluid and serum from TB patients, indicating a relatively higher level of IFN-β protein expression in these samples compared to that in serum from healthy donors (control group). Meanwhile, no significant difference in the level of IFN-β expression was found between pleural fluid and serum from TB patients (P > 0.05) (Supplementary Fig. S1).
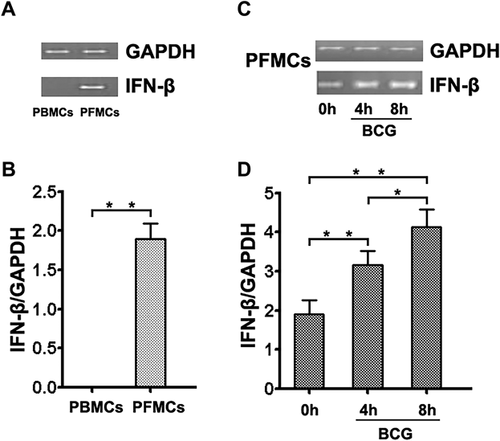
BCG induced IFN-β production
To assess the impact of BCG on IFN-β production, PFMC were treated with BCG at various concentrations and time points. After stimulation, IFN-β production was detected with ELISA, showing that BCG significantly induced the production of IFN-β in a dose-dependent method (Fig. 2a). Moreover, when BCG was applied at 5 µg/mL for 24 hr, the levels of IFN-β were the highest (Fig. 2b). To assess IFN-β expression in different cell subsets, we examined its expression in CD14−and CD14+ cells from PFMC. Notably, IFN-β production was largely confined to CD14+ cells, as CD14−cells expressed low levels of IFN-β. Moreover, IFN-β expression was clearly detected after stimulation with BCG (Supplementary Fig. S2). As the concentration and incubation time of BCG increased, levels of IFN-β tended to decline, perhaps because BCG induced PFMC apoptosis and reduced the number of cells producing IFN-β, resulting in decreased IFN-β production (Supplementary Fig. S3).
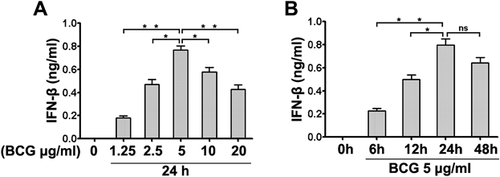
Effects of IFN-β on IL-17 and IFN-γ expression
To analyze the effects of IFN-β on IL-17 and IFN-γ expression, we first detected the expression of IL-17 and IFN-γ in PFMC. Simultaneously, PFMC were harvested after stimulation with BCG or BCG plus IFN-β at different time points. Total cellular RNA was extracted, and IL-17 and IFN-γ expression was detected by RT-PCR. PFMC, but not PBMC (data not shown), spontaneously expressed IL-17 (Fig. 3a) and IFN-γ (Fig. 3c). Levels of IL-17 were markedly reduced, and IFN-γ expression was significantly increased by IFN-β. The graphs show the expression of IL-17 (Fig. 3b) and IFN-γ (Fig. 3d) relative to that of GAPDH.
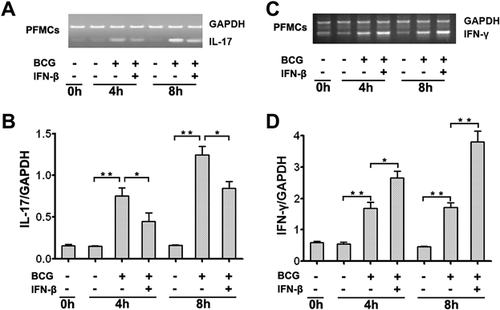
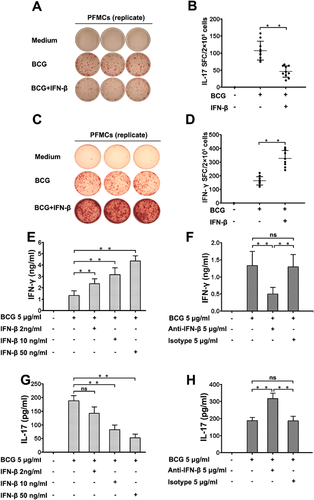
IFN-β regulated IL-17 and IFN-γ production
To investigate how IFN-β mediates IL-17 and IFN-γ in PFMC, we examined whether IFN-β affects the production of IL-17 and IFN-γ at the protein level. In PFMC stimulated with BCG plus IFN-β for 48 hr, IL-17 production was significantly reduced, and IFN-γ production was remarkably increased (Fig. 4c) compared to that in cells stimulated with BCG alone (Fig. 4b,d) (**P < 0.01). To further elucidate the role of endogenous and exogenous IFN-β expression on BCG-induced IL-17 and IFN-γ secretion, we incubated the cells with a purified IFN-β monoclonal antibody and a neutralizing antibody against IFN-β while stimulating PFMC with BCG at 37°C for 48 hr. Our data suggested that exogenous IFN-β markedly enhanced the production of IFN-γ (Fig. 4e) and decreased the levels of IL-17 (Fig. 4g) in a dose-dependent way. Simultaneously, neutralization of IFN-β substantially decreased the levels of IFN-γ and significantly increased the amount of IL-17. By comparison, an isotype-matched control antibody had no effect on IFN-γ and IL-17 (Fig. 4f,h, respectively). In addition, to further explore the local immunoregulatory effects of IFN-β on TB patients and healthy donors, we also evaluated IFN-β regulation of Th1 and Th17 cells from PFMC and PBMC harvested from TB patients and healthy donors. PFMC and PBMCs were stimulated with or without BCG plus IFN-β for 24 hr in the presence of BFA. Cells were first gated for CD3+ T cells and then for CD4+ T cells, and IL-17 and IFN-γ expression levels were evaluated by flow cytometry. IFN-β suppressed the level of IL-17 and increased the production of IFN-γ in both PFMC and PBMC harvested from TB patients and healthy donors (Supplementary Fig. S4).
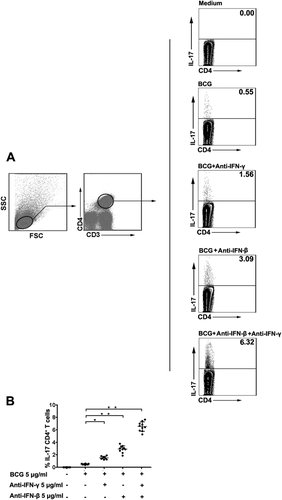
Effects of IFN-β and IFN-γ on IL-17 production
To observe and analyze the roles of IFN-β and IFN-γ in IL-17 cytokine production at the single-cell level, we used flow cytometry to detect the levels of IL-17 in CD4+T cells harvested from PFMC. Independent experiments repeated six times showed that IL-17 secretion was markedly increased when anti-IFN-β and anti-IFN-γ were added to the PFMC cultures after stimulation with BCG at 37°C for 24 hr (Fig. 5).
DISCUSSION
TB is one of three leading causes of death from infectious diseases worldwide and in China is classified as a critical disease. Our previous study indicated that IFN-α is potentially expressed in PFMC 11, but whether IFN-β has a very important immunomodulatory effect during TB infection is currently unknown. Thus, we further investigated the biological function of IFN-β in PFMC harvested from tuberculous pleuritis patients.
IFN comprise a broad spectrum of antiviral agents and are produced mainly from monocytes and lymphocytes in response to various pathogens. According to their specific IFN receptor (IFNR), IFN are divided into types I, II and III. Type I IFN are well known to play important roles in host defense against viruses through activation of IFN receptor A (IFNAR). In addition, IFN-γ, a critical type II IFN, is a canonical cytokine of adaptive Th1 immunity that is essential for immune responses to intracellular bacteria. Type III IFN comprise three members, IFN-λ1, IFN-λ2 and IFN-λ3 12. Traditionally, IFN are cytokines that play important roles in immune responses. IFN antiviral biological activities are presented mostly by inducing the production of antiviral proteins and enhancing the vitality of NK cells, macrophages and T lymphocytes, which may mediate the inhibitory effects of IFN on virus replication. IFN provide the first wave of innate immune defense against virus infections and can also regulate adaptive immune responses by modulating transcriptional activation of IFN-stimulated genes. Some studies have reported the role of IFN-β during virus infection, but its role in tuberculosis pleurisy has not been studied 13. Some studies have shown that leukocytes from active, not latent, TB patients express high levels of IFN-inducible genes, strongly advocating for a contributive effect of type I IFN on TB progression in humans 14. Previous studies have also shown that M. tb infection significantly increases the expression of type I IFN-inducible genes in vitro 15, 16. Nevertheless, the role of IFN-β in the regulation of M. tb infection is not clearly understood. In this article, we initially reported that activated BCG induced IFN-β expression in vivo.
In the present study, we evaluated the distribution and functional characteristics of IFN-β using RT-PCR and ELISA. Five kinds of samples were detected, including PFMC isolated from TB pleural effusions, TB pleural effusion fluid and serum from TB patients, and PBMC and serum isolated from the peripheral blood of healthy subjects. PFMC, TB pleural effusion and serum samples from TB patients consistently expressed IFN-β. However, IFN-β was absent in PBMC and serum from healthy subjects. These results suggest a potential role of IFN-β during TB development. For the first time, we showed that freshly isolated PFMC abundantly express IFN-β in vivo. Moreover, IFN-β mRNA expression was significantly increased in PFMC after stimulation with BCG.
The main role of Th1 cells in defense against TB is presumably as a result of the ability of IFN-γ to activate macrophages and stimulate phagocytosis, phagosome maturation, reactive nitrogen intermediate production, antigen presentation, and autophagy 17-20. These studies support that IFN-γ plays a protective role in anti-TB immunity, but some of the findings are conflicting. For example, some studies showed that when given alone, IFN-γ did not control M. tb infection either in vitro or in vivo 21, 22. In other studies, IFN-γ production was correlated with reduced M. tb loads, but this phenomenon did not reflect the power of protection 23. In addition, some authors reported that IFN-γ plays a protective role by inhibiting a deleterious response in Th17 cells rather than by inhibiting M. tb replication 24. Meanwhile, in some experimental models, CD4+ T cells producing IFN-γ were deleterious 25, 26.
IL-17 is an important cytokine secreted by Th17 cells that plays a crucial role in anti-infective and inflammatory responses. In fact, IL-17 mediates antibacterial and proinflammatory responses, suggesting that its role in the infection process is complex 27, 28. The role of IL-17 responses in humans with TB was mainly evaluated by comparing the responses of TB patients and healthy individuals, but the results of these experiments are contradictory. Some studies reported that the amounts of IL-17-producing cells in TB patients were reduced, suggesting that Th17 contributes to TB protection 29. This view is supported by the findings in another study that low serum levels of IL-17 were associated with high mortality rates in TB patients 30. In addition, some authors reported that blood and bronchoalveolar fluid samples from healthy controls and TB patients showed similar IL-17 levels 31. As discussed above, the relationship between IFN-γ and IL-17 during M. tb infection is complex and not well understood. The vital questions of how IFN-γ interacts with IL-17 and how IFN-β affects the regulation of IFN-γ and IL-17 production during M. tb infection are poorly understood.
To further investigate the function of IFN-β with regard to IL-17 and IFN-γ secretion, we compared IL-17 and IFN-γ expression in PFMC harvested from TB pleural effusions with PBMC from healthy donors under various stimulations using RT-PCR. PFMC, but not PBMC (data not shown), expressed IL-17 and IFN-γ in vivo. Moreover, increased amounts of IL-17 and IFN-γ were observed in PFMC stimulated with BCG. Additionally, exogenous and endogenous IFN-β significantly suppressed IL-17 secretion in PFMC after stimulation with BCG. In contrast, IFN-β markedly increased the production of IFN-γ. Importantly, IFN-β suppressed IL-17 secretion by two distinct mechanisms: IFN-β directly inhibited the levels of IL-17 while up-regulating the levels of IFN-γ, which reduced the abundance of IL-17. Our findings indicate that mycobacteria use different pathways for regulating IL-17 secretion, and in the context of M. tb infection, the induction of IFN-β and IFN-γ is associated with M. tb disease progression in infected patients. In our previous study, we reported that PFMC expressed high levels of the cytokines IFN-γ, IL-22 and IL-17 after stimulation with BCG as determined by mRNA and protein expression analysis 10. Results from our previous studies indicated that BCG induces the production of IFN-γ, IL-2 and TNF-α in cultured supernatants and cultured CD4+ T cells from PFMC 32, 33. The present study is a continuation of our previous research, and the data are supported in part by our previous work.
In this study, IFN-β enhanced IFN-γ production in PFMC. In fact, IFN-β showed both protective and counterprotective functions during disease infection, showing its various regulatory effects on IFN-γ. A recent study showed that IFN-β suppressed IFN-γ responses in Mycobacterium leprae infection 34. However, another study on Listeria monocytogenes infection found a type I IFN that acts directly on CD4+ T cells, resulting in IFN-γ production 35. Another study also showed that IFN-α/β can provide the third signal directly to CD8+ T cells through a STAT4-dependent pathway for cell survival, cytolytic function development, and IFN-γ production 36. In addition, our recent work showed that IFN-α induced IFN-γ production in PFMC during BCG stimulation 11. Different kinds of bacteria or bacteria of the same type with different pathogenicities and immune responses are presumably attributed to discrepancies in the effects of IFN-β on IFN-γ. These data also partly support our observation that IFN-β enhances the levels of IFN-γ during BCG stimulation.
In summary, for the first time, we systematically analyzed the production of IFN-β and its biological function in tuberculous pleural effusion patients. High levels of various cytokines were detected in PFMC freshly isolated from tuberculosis pleural effusions, indicating the regulatory effect of IFN-β on IL-17 and IFN-γ production. These findings not only provide a new theoretical approach for evaluating the immune responses to M. tb for immune escape but also provide a new direction for the clinical treatment of TB.
ACKNOWLEDGMENTS
We acknowledge the patients and healthy volunteers for their blood donation contributions to this study.
DISCLOSURE
The authors have no financial conflicts of interest.