In vitro antibiofilm and anti-adhesion effects of magnesium oxide nanoparticles against antibiotic resistant bacteria
ABSTRACT
The aim of the current investigation was to determine the antibacterial and antibiofilm potential of MgO nanoparticles (NPs) against antibiotic-resistant clinical strains of bacteria. MgO NPs were synthesized by a wet chemical method and further characterized by scanning electron microscopy and energy dispersive X-ray. Antibacterial activity was determined by broth microdilution and agar diffusion methods. The Bradford method was used to assess cellular protein leakage as a result of loss of membrane integrity. Microtiter plate assay following crystal violet staining was employed to determine the effect of MgO NPs on biofilm formation and removal of established biofilms. MIC values ranged between 125 and 500 μg/mL. Moreover, treatment with MgO NPs accelerated rate of membrane disruption, measured as a function of leakage of cellular proteins. Leakage of cellular protein content was greater among gram-negative bacteria. Cell adherence assay indicated 25.3–49.8% inhibition of bacterial attachment to plastic surfaces. According to a static biofilm method, MgO NPs reduced biofilm formation potential from 31% to 82.9% in a time-dependent manner. Moreover, NPs also significantly reduced the biomass of 48, 72, 96 and 120 hr old biofilms (P < 0.05). Cytotoxicity experiments using a neutral red assay revealed that MgO NPs are non-toxic to HeLa cells at concentrations of 15–120 μg/mL. These data provide in vitro scientific evidence that MgO NPs are effective and safe antibiofilm agents that inhibit adhesion, biofilm formation and removal of established biofilms of multidrug-resistant bacteria.
Abbreviations
-
- EDX
-
- energy dispersive X-Ray
-
- MBC
-
- minimum bactericidal concentration
-
- MgCl2
-
- magnesium chloride
-
- MgO
-
- magnesium oxide
-
- MIC
-
- minimum inhibitory concentration
-
- NaNO3
-
- sodium nitrate
-
- NaOH
-
- sodium hydroxide
-
- NP
-
- nanoparticle
-
- ROS
-
- reactive oxygen species
-
- SEM
-
- scanning electron microscope
Emergence of antibiotic resistance is an increasingly important public health issue. During the last few decades, microbial infections have become a serious threat to public health because of rapid development of resistant bacterial strains, thus challenging clinical treatment globally 1. These newly resistant strains have led to failure of bacteria to respond to standard antimicrobial treatment, resulting in persistence of disease, higher health expenditures and increased risk of death 2. Although various new antibiotics have been marketed in the last few decades, none of them have shown enhanced activity against multidrug-resistant bacteria. Thus, development of alternative therapeutic agents for coping with the problems associated with these drug-resistant pathogens is urgently needed.
In the last couple of decades, NP technology has become available and enabled development of reliable broad-spectrum NP-antimicrobial agents. In addition, NPs have the capacity to extend the limits of traditional antimicrobial agents 2. NPs are highly reliable antibacterials that act in a complementary way to antibiotics; they are therefore gaining wider interest because of their potential to fill the gaps where antibiotics mostly fail, including dealing with multidrug-resistant mutants and biofilms 3. Bacteria growing as structured aggregates or biofilms are better able to survive harsh environmental conditions and are resistant to antibiotic treatment and host immunity 4. Biofilms are consortiums of single or multiple species of bacteria lodged in an extracellular polymeric substance that is mainly constituted of proteins, polysaccharides and nucleic acids 5. The ineffectiveness of antibiotics in treating biofilm infections, which is attributable to a permeability barrier, may cause problems in the ultimate eradication of diseases 6. Therefore, novel antibiofilm agents are required to treat infections caused by biofilm-producer pathogens.
Inorganic metal oxide NPs are efficient and promising antimicrobial agents because of their activity at low concentrations and their biocompatibility with mammals 7. MgO NPs reportedly have antibacterial potential against both gram-negative 8, 9 and gram-positive bacteria such as Staphylococcus aureus 10. However, the role of MgO NPs as efficient biofilm inhibitors has not yet been given sufficient attention.
The present study was therefore designed to synthesize and characterize MgO NPs and to assess their antibacterial and antibiofilm potential against antibiotic-resistant bacteria. A static biofilm assay was used to determine the effect of sub-inhibitory concentrations of MgO NPs on biofilm formation and mature biofilms. Furthermore, the effects of sub-inhibitory concentrations of MgO NPs on bacterial attachment to plastic surfaces and leakage of cellular proteins were also elucidated. Additionally, the cytotoxic effect of MgO NPs was evaluated against HeLa cells using a neutral red uptake assay.
MATERIALS AND METHODS
Bacterial strains and reagents
Three antibiotic resistant strains isolated from urine and pus samples, namely Escherichia coli (Accession No. KT273995) and Klebsiella pneumoniae (Accession No. KT273996) from urine specimens and S. aureus (Accession No. KT250728) from a pus sample were used in the present study. All strains were grown in LB broth and on LB agar (supplemented with 1.5% agar) under standard conditions. All chemicals used in the present study were obtained from Sigma–Aldrich (St. Louis, MO, USA) and Merck (Darmstadt, Germany) unless otherwise stated. For synthesis of MgO NPs, MgCl2, NaOH and NaNO3 were used as progenitors and diethylene glycol as a stabilizing agent.
Synthesis of MgO NPs
Magnesium oxide NPs were prepared by a wet chemical synthesis method following the protocol of Sundrarajan et al. 11 with minor modifications. Briefly, an aqueous solution of 21 mmol NaOH (pH ranging from 12 to 13) and 8.8 mmol NaNO3 (pH, 6.8) were mixed thoroughly followed by addition of 100 mmol MgCl2 (pH, 6.8) solution. The final solution was initially mixed at room temperature for 30 min followed by mixing at 90°C for 18 hrs. Finally, the mixture was allowed to cool at room temperature, after which white precipitates were collected and washed with deionized water and analytical grade absolute methanol by centrifugation. The obtained white-colored magnesium hydroxide NPs were dried at 90°C for 6 hrs to yield MgO NPs.
Characterization of MgO NPs
For characterization of MgO NPs, SEM and EDX spectroscopy were used. EDX analysis was performed to confirm the elemental composition of MgO NPs. For characterization of MgO NPs, dried particles were mounted on aluminum stubs and coated with gold to obtain better contrast. Analysis was performed by SEM (Nova NanoSEM 450; FEI, Holand) equipped with an Oxford EDX microanalysis system (Oxford Instruments, Singapore).
Bacterial susceptibility assay
The susceptibility of bacteria to MgO NPs was determined by an agar diffusion method 12. Bacterial inocula were made by diluting overnight cultures of each strain to get a final density of 5 × 105 CFU/mL; these were plated on LB agar medium. Wells of 5 mm diameter were made aseptically on LB agar plates and 0.1 mL of various concentrations of MgO NPs (2000 μg/mL, 1000 μg/mL and 500 μg/mL) were dispensed into separate wells followed by overnight incubation at 37°C. After incubation, diameters of zones of inhibition were recorded as a function of bacterial susceptibility. Wells containing DMSO alone served as a control.
Measurement of bacteriostatic and bactericidal potential
The antibacterial activity of MgO NPs was determined by a broth microdilution method followed by cell viability determination using a dye-exclusion assay. Briefly, two-fold broth microdilutions of NPs in Mueller–Hinton broth were made using 96-wells microtiter plate (Thermo Fisher Scientific, Rockford, IL, USA). To assess bacteriostatic potential (MIC) of MgO NPs, target strains were exposed to 0–1000 μg/mL of NPs prepared in 0.1% (v/v) of DMSO. Adjusted inocula were dispensed into the wells at a final bacterial density of 5 × 105 CFU/mL and the plates incubated at 37°C for 24 hrs. Experiments were performed in triplicate and positive and negative controls were also included. After incubation, absorbance was recorded at 578 nm to determine MIC values. Cell viability was also assessed by changes in color from yellow to blue after addition of a methanolic solution of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliium bromide (5 mg/mL). To investigate the bactericidal potential (MBC). 10 μL of the suspension was collected from wells with no visible growth (MIC or higher concentrations) and plated onto LB agar plates. The plates were incubated at 37°C for 24 hrs and the MBC determined.
Cellular protein leakage determination
Disintegration of cell envelopes was measured by quantification of cellular protein leakage as a function of cell death. For estimation of cellular protein leakage, bacterial inocula at a final density of 5 × 105 CFU/mL were added to LB medium of various concentrations (0.5 × MIC and 1 × MIC respectively for each strain) of MgO NPs. Cells grown in LB medium without NPs were employed as control. Each culture was placed on a shaking incubator for 12 hrs at 37°C. After incubation, 1 mL from each culture was harvested by centrifugation for 30 min (300 g, 4°C) and the collected supernatant used for to quantitate protein using the Bradford assay and taking absorbance at 595 nm 13.
Determination of cell viability using sub-inhibitory concentrations of MgO NPs
The effects of sub-inhibitory concentrations of MgO NPs on the viability of antibiotic resistant bacteria were determined by growing a cell suspension of 5 × 105 CFU/mL in LB broth with 0.5 × MIC of MgO NPs. A control without NPs but with the same density as the bacterial inocula was included. The cultures were incubated at 37°C. After 0, 4, 8, 12 and 16 hrs, 0.1 mL aliquots from each sample were taken, serially diluted and spread on LB agar plates to determine the number of viable cells after 24 hrs of incubation.
Effect of MgO NPs on adherence of bacteria to plastic surfaces
The impact of sub-inhibitory concentrations of MgO NPs on the adherence of bacteria to the surface of 96-wells microtiter plate was determined following the method of Rodrigues et al. 14. Briefly, overnight culture of each strain (standardized to 0.5 McFarland) in LB medium was diluted with fresh LB medium (1: 20) with and without sub-inhibitory concentrations (0.5 × MIC) of MgO NPs, after which 200 μL of this suspension was dispensed to the wells of microtiter plate and incubated for 12 hrs at 37°C. Following incubation, the unbound cells were removed by washing each well three times with 0.85% NaCl. Cells adherent to the wells were stained with 0.1% crystal violet for 10 min followed by elution with 200 μL of 33% glacial acetic acid after washing with 0.85% NaCl. To quantify the number of cells attached to the wells of the polystyrene plates, absorbance at 546 nm was measured. Negative (sterile growth medium) and positive (working solution) controls were included.
Biofilm formation assay
To evaluate the effect of MgO NPs at sub-inhibitory concentrations on biofilm formation, biofilm formation was quantified using the method of Christensen et al. 15 with some modifications. Overnight grown cultures of target bacteria were diluted with fresh LB broth (1:20) with and without sub-inhibitory concentrations of MgO NPs. This suspension (200 μL) was dispensed into 96-well microtiter plates (Thermo Fisher Scientific) and incubated at 37°C for 48, 72, 96 and 120 hrs without agitation. Following incubation, the medium was decanted and the wells washed thoroughly with saline (0.85 % NaCl). Biofilms were quantified by staining the attached cells with 0.1% crystal violet for 15 min. The wells were washed thoroughly with saline to remove excessive dye, whereas crystal violet-attached cells were solubilized in 200 μL of glacial acetic acid (33%) and the absorbance determined at 546 nm with a microtiter plate reader (Bio-Rad Microplate Reader, 680XR; Bio-Rad, Hercules, CA, USA). Negative (sterile growth medium) and positive (working solution) controls were also included in the experiment.
Effect of MgO NPs on established biofilms
Effects of MgO NPs on established biofilms were assessed following Lellouche et al.'s method 16 with minor modifications. Briefly, overnight cultures of each bacterial strain grown in LB medium were diluted with fresh medium (1:20). The suspension (200 μL) was then dispensed into 96-well microtiter plates and incubated at 37°C for 48, 72, 96 and 120 hrs without agitation. After incubation, the wells were washed three times with 200 µL of normal saline to eliminate non-adherent cells. Wells containing attached biofilms were treated with sub-inhibitory concentrations of MgO NPs and the plates incubated overnight at 37°C; untreated cells served as control. After incubation, the adherent cells were stained with 200 μL of 0.1% (v/v) crystal violet for 15 min at room temperature. The wells were washed thoroughly with 0.85% NaCl to remove excessive dye, after which crystal violet-attached cells were eluted in 200 μL of glacial acetic acid (33%) and the absorbance determined at 546 nm.
Cytotoxic potential of MgO NPs
To check the cytotoxic potential of MgO NPs, a neutral red uptake assay using HeLa cells and following the method of Repetto et al. 17 was employed. HeLa cells were cultured in Dulbecco's modified Eagle's high glucose medium (Gibco, Gaithersburg, MD, USA) with 10% FBS and 1% penicillin/streptomycin (Gibco) as 15,000 cells per cm2 and incubated for 24 hrs at 5% CO2 and 90% humidity. After incubation, the medium was changed and the cells incubated again for 24 hrs with various concentrations of MgO NPs (0–240 µg/mL) along with control (without any additive). Following incubation, media were removed and the cells washed with PBS, after which 100 µL neutral red media (40 µg/mL) was added and the cells incubated again at 37°C for 2 hrs. Following incubation, neutral red media was removed and the cells were again washed with PBS and destained with 150 µL of freshly prepared de-staining solution (49% water, 50% absolute ethanol and 1% glacial acetic acid). A microtiter plate shaker was used to shake the microtiter plates for 10 min. Absorbance was measured at 540 nm using a microtiter plate reader.
Microscopic examination of HeLa cells
HeLa cells growing in the presence of various concentrations of MgO NPs (0–240 µg/mL) were examined morphologically using a phase contrast microscope. Digital images of control and treated cells were obtained with an attached Charge-Coupled Device (CCD) SP 480 H color camera (Olympus, Tokyo, Japan).
Statistical analysis
All experiments were performed in triplicate and the results are presented as means ± SE. The significance of the results of each experiment was checked by Student's t-test and anova using Microsoft, Excel. P < 0.05 was considered to denote statistical significance.
RESULTS
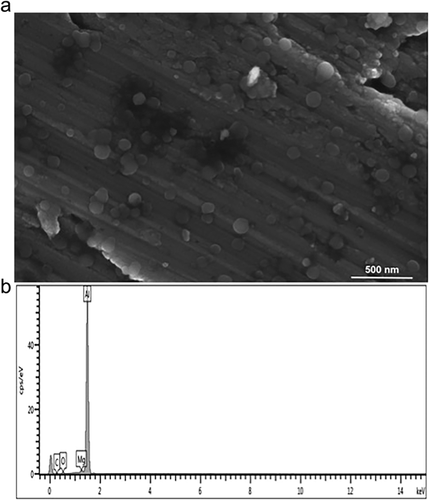
The yield of MgO NPs using the wet chemical method was 340 mg. The product was white, crystalline and odorless with a pH of 6–7. SEM images of the prepared MgO NPs revealed that most of them were of spherical shape, <100 nm isn size and with average diameter of 50–70 nm, as shown in Figure 1a. EDX spectroscopy indicated the presence of magnesium and oxide elements, thus confirming the purity of the synthesized product (Fig. 1b).
The effects of various concentrations of MgO NPs were also determined by an agar well diffusion method and it was found that NPs exhibited antibacterial potential in a dose-dependent manner, the diameters of the zone of inhibition increasing with increasing concentrations of NPs and ranging from 17.5 to 35.5 mm. The largest zone of inhibition was observed against K. pneumoniae KT273996 (35.5 mm), followed by E. coli KT273995 (28 mm), as shown in Table 1.
| Diameter of zone of inhibition (mm) | |||
|---|---|---|---|
| Bacterial strain | MgO NPs (2000 μg/mL) | MgO NPs (1000 μg/mL) | MgO NPs (500 μg/mL) |
| Escherichia coli (KT273995) | 28 ± 0.33 | 21 ± 0.288 | 17.5 ± 0.288 |
| Klebsiella pneumoniae (KT273996) | 35.5 ± 0.33 | 33.5 ± 0.33 | 30.5 ± 1.90 |
| Staphylococcus aureus (KT250728) | 25.5 ± 0.268 | 23.5 ± 1.32 | 20.5 ± 2.18 |
- Diameters of zones of inhibition are expressed as mean ± SE.
To evaluate the susceptibility of antibiotic-resistant bacteria to MgO NPs, MICs and MBCs were determined by a broth microdilution method. It was found that MICs ranged between 125 and 500 μg/mL. For K. pneumoniae KT273996, the MIC of MgO NPs was found to be 125 μg/mL. MBC values ranged between 500 and 1000 μg/mL, the values being two- to three-fold those of their respective MICs (Table 2).
| Strains | MIC (μg/mL) | MBC (μg/mL) |
|---|---|---|
| E. coli (KT273995) | 250 | 500 |
| K. pneumoniae (KT273996) | 125 | 500 |
| S. aureus (KT250728) | 500 | 1000 |
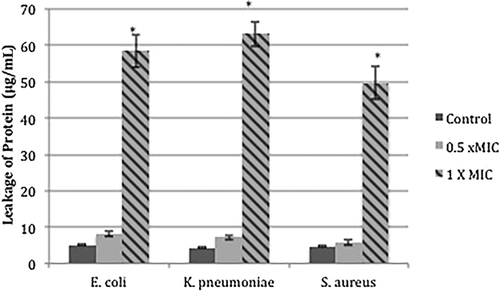
To determine the effect of MgO NPs on leakage of cytoplasmic proteins, the amount of protein released into the supernatant was estimated using the Bradford method. It was found that cells treated with MgO NPs (at 1 × MIC leaked significantly more protein than controls and cells treated with sub-inhibitory concentrations (0.5 × MIC) of NPs (***, P < 0.001) (Fig. 2). The large amounts of protein released into the supernatant after 12 hrs incubation of cells in the presence of NPs, indicates that cell membranes had been disrupted. However, no significant difference in protein leakage were observed for control cells or cells treated with sub-inhibitory concentrations of NPs (P > 0.05). Protein leakage was greater in the case of gram-negative bacteria such as K. pneumoniae KT273996 (63.14 μg/mL) and E. coli KT273995 (58.4 μg/mL) than for gram-positive ones such as S. aureus KT250728 (49.7 μg/mL), as shown in Figure 2.
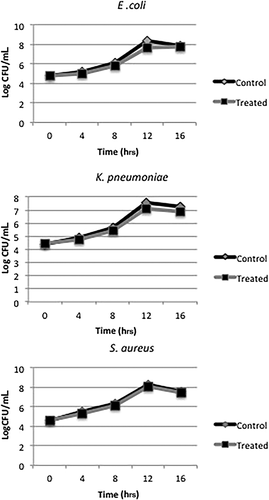
To determine the effects of sub-inhibitory concentrations of MgO NPs on viability of bacterial strains, cells were grown in the presence and absence of sub-inhibitory concentrations of NPs relevant to each strain for 16 hrs. After incubation, CFUs was counted after harvesting the cells at different time intervals and determining the number of viable cells after 24 hrs. There was no significant difference in number of viable control cells and those treated with sub-inhibitory concentrations of MgO NPs (P > 0.05), suggesting that growth of cells was not significantly affected by the presence of NPs (at 0.5 × MIC) (Fig. 3).
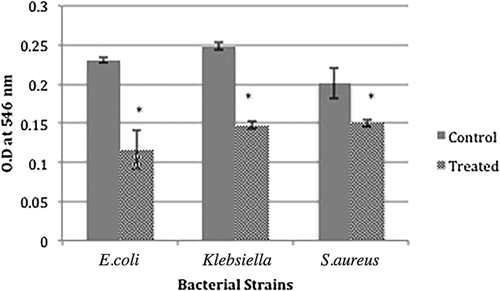
A cell attachment assay was performed to determine the effect of MgO NPs at sub-inhibitory concentrations on bacterial adherence to plastic surfaces. It was found that bacterial attachment to the surface of plastic was significantly less after 12 hr of incubation in the presence of MgO NPs than for control cells (***, P < 0.001). There was 25.3%–49.8% inhibition of attachment by NPs, as shown in Figure 4. However, the anti-adhesion potential of MgO NPs did not differ significantly (P > 0.05) between the tested strains.
Biofilm was quantitated using a static biofilm assay to determine the effects of sub-inhibitory concentrations of MgO NPs on the biofilm-forming capacity of antibiotic-resistant bacteria. As shown in Figure 5a, the biofilm-forming capacity was significantly less for cells treated with NPs, being 31%–82.9% of that for control cells (**, P < 0.01). However, for most time intervals treatment with MgO NPs did not significantly reduce the biofilm biomass (P < 0.05). Biofilm formation by S. aureus KT250728 was reduced to 82.89% and by E. coli KT273995% to 82.12% after 96 hrs of incubation (Fig. 5a).
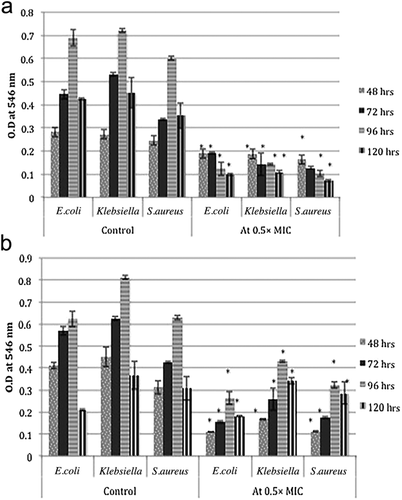
The effects of MgO NPs on already established and mature biofilms were determined by microtiter plate assay at sub-inhibitory 0.5 × MIC concentrations; the results are shown in Figure 5b. The biomass of established biofilms (biofilms established after 48, 72, 96 and 120 hrs) of cells treated with MgO NPs was significantly less than that of controls (**, P < 0.01). Additionally, over time NPs showed less ability to decrease biofilm viability: there was only a 7.06%–14.21% decrease in biomass of mature biofilms at 120 hr as compared with 48 hr old biofilms (Fig. 5b).
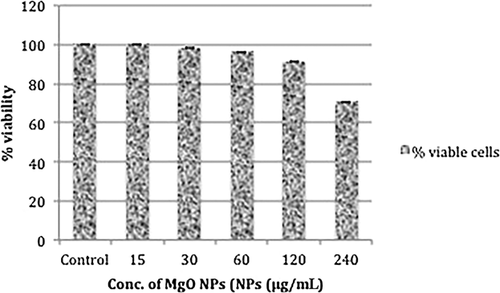
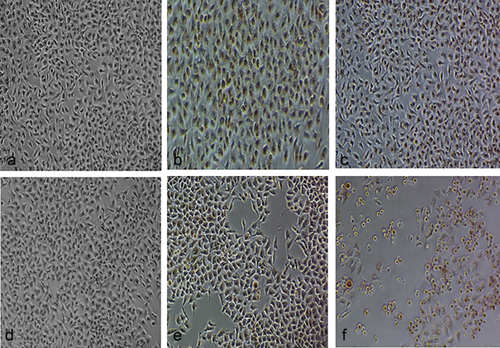
The viability of HeLa cells was determined by neutral red uptake assay after incubating them in the presence of different concentrations of MgO NPs for 24 hrs; the results were quantified in terms of % cellular viability. There was no significant change in % viability of HeLa cells at the various concentrations tested: cellular viability was found to be 91.16%–100% up to concentrations of 120 μg/mL as compared with untreated cells (100% viability) (P > 0.05). At the highest concentration tested (240 μg/mL), around 70.5% of cells were viable, as shown in Figure 6. Phase-contrast microscopy showed that MgO NPs are non-toxic to HeLa cells at the concentrations used (15–120 μg/mL). However, NPs did have cytotoxic effects at the highest concentration (240 μg/mL), as shown in Figure 7, various morphological changes, including cell rounding, detachment and lysis, having been observed. Toxic concentrations of NPs resulted in extensive vacuolation followed by cell rounding, detachment from the substratum, and finally cellular lysis.
DISCUSSION
The present study provides experimental proof that MgO NPs can be utilized as a promising and efficient biofilm inhibitor against targeted antibiotic-resistant bacteria. We synthesized spherical NPs of average size 50–70 nm by a wet chemical method. However, in a previous study in which a urea-based combustion method was used, the average crystalline size of MgO was 15–60 nm with particle sizes of 300–350 nm as determined by SEM micro-imaging 18. According to our MIC and MBC data, MgO NPs exhibited antibacterial activity against target bacterial strains. Previous studies have also shown that nano-MgO particles are effective therapeutic agents against various pathogens 9, 10, probably as a result of their numerous crystal defects, large surface area and electrostatic interactions with bacterial envelopes 19, 20. Metal oxide nanoparticles are capable of increasing membrane permeability, thus facilitating leakage of cytoplasmic contents. In the current study, we found that MgO NPs significantly enhanced cytoplasmic leakage by destabilizing the permeability of cell membranes. Moreover, protein leakage was more pronounced for gram-negative bacteria such as E. coli KT273995 and K. pneumoniae KT273996 than for gram-positive S. aureus. These variations may be attributable to differences in peptidoglycan and membrane lipid content. Our results are consistent with those of previous studies, which have documented greater protein leakage with gram-negative than gram-positive bacteria after treatment of cells with silver NPs 13, 21.
It has been observed that concentrations above the MIC need to be reached between consecutive doses for antibiotics to be effective. A certain interval after administration, an antibiotic's concentration within tissues decreases below the MIC, this being known as a sub-inhibitory concentration of the antibiotic. Sub-inhibitory concentrations of chemotherapeutic agents are inefficient in killing bacteria, but have the potential to change various attributes, such as the physical and chemical nature of cell surfaces, and to modulate expression of potent virulence factors 22, 23. We found that sub-inhibitory concentrations of MgO NPs did not alter cell viability much as compared with controls. We therefore tested sub-inhibitory concentrations of MgO NPs against selected bacteria to determine their effect on various virulence variables such as adhesion and biofilm formation.
Adherence is a complex process involving multiple biological and physiochemical variables that control the colonization of bacteria to abiotic surfaces 24, 25. Our study indicated that MgO NPs reduce bacterial adherence to plastic surfaces at sub-MIC levels. Given that adherence and colonization on various surfaces are preliminary steps toward development of infection and biofilm formation, inhibiting the critical step of adherence may enable prevention of the remaining stages of biofilm formation.
Biofilm-forming microbes have unique potential to cause infection. According to a report from the National Institutes of Health and Centre of Disease Control, approximately 65%–80% infections are caused by biofilm-forming microbes, E. coli, Pseudomas aeruginosa and S. aureus being major culprits 26. Our study revealed that MgO NPs at sub-inhibitory concentrations inhibit the biofilm-forming potential of pathogens such as E. coli, K. pneumoniae and S. aureus, which are known for their biofilm-forming capacity. We found that MgO NPs reduced the biofilm-forming capacity of bacteria by 31%–82.9% in a time-dependent manner, comparable to previous studies on silver NPs 27. However, the concentration of Ag-NPs used in that study was higher than the concentration of MgO NPs used in the current study. Another study on the antibiofilm potential of zinc oxide nanoparticles against Rothia species (opportunistic pathogens in the oral cavity) reported observing 47%–69% biofilm inhibition at concentrations of 200–300 μg/mL 28. However, in contrast to these observations, Haney et al. reported an increase in biofilm biomass of P. aeruginosa at a concentration of 0.2 mg/mL of superparamagnetic iron oxide NPs 29.
We also studied the effects of MgO NPs on removal of already established biofilms at different time intervals by applying sub-inhibitory concentrations of NPs. We found that MgO NPs at sub-MICs not only inhibit biofilm formation at different time intervals, but also reduce the number of cells attached in established biofilms. Lellouche et al. have reported that magnesium fluoride NPs have an inhibitory effect on established biofilms of E. coli and S. aureus 16. In contrast, Khiralla and El-Deeb have reported the inhibitory potential of selenium nanoparticles on biofilm formation by S. aureus and P. aeruginosa; however, various concentrations of selenium nanoparticles did not remove already established biofilms 30.
The cytotoxic potential of various antibacterial agents is a major concern regarding their development and utilization as chemotherapeutic agents. In the current study, we evaluated the cytotoxicity of MgO NPs against HeLa cells and found that NPs remained non-toxic up to a concentration of 120 μg/mL1. Ge et al. have reported that MgO NPs remain non-toxic at a low concentration (0.3 mg/mL) 31. At a higher concentration (240 μg/mL), the NPs were toxic and led to morphological abnormalities and cell death. The observed cellular deformities included extensive vacuolation, contact loss, cell rounding and lysis. These cellular alterations may have been attributable to reactiveness of metal cations and generation of ROS 32. These researchers’ findings indicate that at higher concentrations NPs react with biomolecules and produce ROS, leading to the cell death.
In the present study, we have clearly demonstrated the role of MgO NPs as an efficient anti-adherent and antibiofilm agent at sub-inhibitory concentrations. Biofilm formers are highly resistant to various antimicrobial agents and have thus generated a serious public health concern. In the current study, we tested only one strain per species; therefore, in the future there is a need to test other species to enable effective use of MgO to treat infections caused by antibiotic-resistant biofilm formers.
DISCLOSURE
No financial contributions were received from any organization. The authors declare they have no conflict of interest.