A discrete choice experiment on oral and injection treatment preferences among moderate-to-severe psoriasis patients in Japan
Abstract
Long-term psoriasis (PsO) management remains challenging. With growing variation in treatment efficacy, cost, and modes of administration, patient preferences for different treatment characteristics are not well understood. A discrete choice experiment (DCE), informed by qualitative patient interviews, was conducted to assess patient preferences for different attributes of PsO treatments; 222 adult patients with moderate-to-severe PsO receiving systemic therapy participated in the DCE web survey. Better long-term efficacy and lower cost were preferred (preference weights p < 0.05). Long-term efficacy had the highest relative importance (RI) and mode of administration was as important as the outcome attributes (efficacy and safety). Patients also preferred oral to injectable administration. In subgroup analyses by disease severity, residence, psoriatic arthritis as a comorbidity, and gender, the trends for each subgroup were the same as the overall population although the extent of RI for administration mode varied. Mode of administration was more important for patients with moderate versus severe disease, or rural versus urban residence. This DCE utilized attributes related to both oral and injectable treatment as well as a broad study population of systemic treatment users. Preferences were further stratified by patient characteristics to explore trends in different subgroups. Understanding the RI of treatment attributes and the attribute trade-offs acceptable to patients helps inform moderate-to-severe PsO systemic treatments decisions.
1 INTRODUCTION
Psoriasis (PsO) is a chronic inflammatory skin disease in which approximately 90% of patients are characterized by well-demarcated and erythematous plaques covered with silvery scales.1 The prevalence of PsO ranges from 0.09% to 11.4% worldwide, with an estimated 100 million people affected.2, 3 A claims database study using data from 2010 to 2011 reported that more than 400 000 people are living with PsO in Japan, with a prevalence estimated to be 0.34%.4
The physical, economic, and psychological burdens of PsO are substantial.5 This disease negatively affects social functioning, including decreased work efficiency and distress at work due to skin symptoms such itching or redness.6 Occupational impairment leads to lower wages or barriers to promotion, further exacerbating the psychosocial burden of PsO.7 Additionally, a large proportion of patients experience stigma and discrimination, which is exacerbated for moderate-to-severe PsO patients as the severity of the disease can impact self-esteem negatively and contribute to greater social isolation.8-10
Without any curative treatments available, symptom control is the primary goal of PsO management.3 Treatments for PsO can be classified as topical therapies, phototherapy, oral medications, and biologic agents. The choice of treatment type depends on the severity of the disease. The majority of patients with PsO have limited lesions (body surface area <5%) that can be treated with topical therapies, whereas the minority of patients, who have moderate-to-severe PsO, may require systemic therapies including oral medications and biologics.11, 12 In Japan, such biologics have been available since 2010;13 as of May 2022, there are eleven biologics indicated for the treatment of PsO in Japan. Annual epidemiologic surveys conducted by the Japanese Society for Psoriasis Research reported that 26.6% of PsO patients newly diagnosed between 2013 and 2018 received oral medications, and 18.6% were treated with biologics.12 The number of patients who receive topical therapies has been in decline in recent years due to the increased availability of systemic therapy options.12 However, in Japan, there is no treatment guideline for PsO therefore no established treatment algorithm exists. Dispensation of biologic therapy is limited to Japanese hospitals accredited to administer these specific treatments,13 and the costs of biologics are higher than topical or oral medications.14
For PsO treatment more broadly, patient treatment preferences are often not fully discussed in routine clinical practice, and there is a lack of understanding of patient concerns or needs.15 The DCE approach assumes a set of attributes describing key features of treatments and the relative value of a particular treatment to an individual is a function of these attributes. This method has been utilized in multiple disease areas to quantify preferences of patients, caregivers, physicians, and other stakeholders.16-19 In the Japanese setting, two discrete choice experiments (DCEs) have been conducted to identify important treatment attributes from the perspective of moderate-to-severe PsO patients.20, 21 However, these studies included attributes and levels only relevant to biologics and were created and selected by clinicians. Therefore, there remains a lack of evidence from real-world settings in key treatment attribute choices and preferences reflecting all systemic options, including oral medication among Japanese patients with moderate-to-severe PsO.
This study uses a DCE to assess the relative importance (RI) of attributes of both oral and biologic systemic therapies from the patient's perspective when selecting treatments for moderate-to-severe PsO.
2 METHODS
2.1 Development of DCE
The initial vignettes of the DCE were developed based on a review of package inserts of systemic treatments, Japanese guidance for use of biologics for PsO and relevant clinical trials, literature that discusses important PsO treatment attributes from the patient's perspective, and expert opinion. Attributes for potential vignettes included efficacy, safety, mode of administration, and cost related items (Table 1). Characteristics of both oral medications and biologics were incorporated into the draft attributes. Ranges for levels of each potential attribute were derived from the available clinical data and new biologic and oral medications for moderate-to-severe PsO.
| Attribute | Definition | Levels |
|---|---|---|
| Short-term efficacy (PASI75) | Proportion of patients with more than 75% improvement in PsO symptoms (such as erythema, scaling, etc.) within 1 month after starting treatment |
5% (5 out of 100 patients) 10% (10 out of 100 patients) 30% (30 out of 100 patients) |
| Long-term efficacy (PASI75) | Proportion of patients with more than 75% improvement in PsO symptoms (such as erythema, scaling, etc.) at 1 year after starting treatment |
45% (45 out of 100 patients) 65% (65 out of 100 patients) 95% (95 out of 100 patients) |
| Side effects (infections) | Percentage of patients who develop infections (pneumonia, sepsis, etc.) requiring hospitalization within 1 year of starting treatment |
1% (1 out of 100 patients) 2% (2 out of 100 patients) |
| Side effects (GI-related) | Percentage of patients who develop diarrhea during treatment |
1% (1 out of 100 patients) 5% (5 out of 100 patients) 15% (15 out of 100 patients) |
| Convenience | Administration method and frequency |
Oral: Once daily Oral: Twice daily Hypodermic injection: Every 12 weeks |
| Cost | Monthly out-of-pocket medical expenses (all inclusive) |
Monthly 18 000 JPY Monthly 19 000 JPY Monthly 28 000 JPY |
- Note: Levels are based on package inserts and relevant clinical trial endpoints.
- Abbreviations: GI, gastrointestinal; PASI75, Psoriasis Area and Severity Index 75; PsO, psoriasis.
Subsequently, interviews were conducted with patients who had moderate-to-severe PsO to facilitate attribute development. The interview participants (n = 10) were recruited using a specialized patient recruiting agency in Japan. The participants were selected if they had a clinical diagnosis of moderate-to-severe and were taking oral medications or biologics as systemic therapy. Written consent was obtained prior to all interviews. Each 1-hour, semi-structured interview consisted of two parts. Interviews were audio-recorded and recordings were transcribed, and transcripts were analyzed using ATLAS.ti software.
The first part of the interview consisted of concept elicitation to understand important attributes of PsO treatment from the patient's perspective. Patients were asked about their treatment goals, reasons for switching from previous treatments, desired changes to aspects of their current treatment, key treatment attributes and risks to be avoided. Any spontaneous reports of preferences were also noted. The second part of the interview included a cognitive debriefing where “think aloud” and “verbal probing” procedures were used to assess understanding of the DCE task, its wording, and the relevance of the initial DCE vignettes.
2.2 Design of DCE choice tasks
A block design was implemented to construct choice tasks based on D-efficiency to maximize efficiency of the design.22, 23 First, any strictly dominated tasks (all levels of one alternative are superior to the levels of the second alternative) or implausible choice tasks were excluded in the design to minimize illogical responses. Then, D-efficiency of the resulting possible combinations was maximized to yield a design consisting of 36 choice tasks, which was blocked into three sets of 12 choice tasks (D-efficiency = 92.3). Lastly, one dominant task was added using the highest efficacy levels with the lowest levels of cost and side effects compared to the lowest efficacy levels with the highest levels of cost and side effects to assess whether patients would select the dominant choice as a rationality test. Each block contained 13 choice tasks, and each participant was randomly assigned to only one of these blocks (Figure 1).
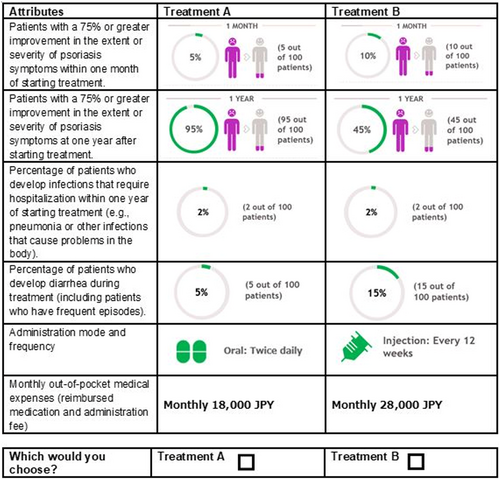
2.3 Participants in the DCE survey
The survey population consisted of self-reported moderate-to-severe PsO adult participants (age ≥20 years) in Japan taking systemic PsO treatment (biologic or non-biologic) at the timing of the survey. Those who reported a diagnosis of guttate, inverse, pustular, erythrodermic, or drug-induced PsO were excluded. Participants were identified through the largest Japanese online patient panel, with approximately 280 000 registered patients operated by Macromill, Inc. Invitations were sent to potential respondents via email. After respondents agreed to participation in the survey and completed an online informed consent, they completed the online DCE survey and a demographics questionnaire, which included age, gender, self-reported severity, time since PsO diagnosis, current and previous PsO treatment, comorbid conditions, height, weight, education, employment status, residence, and level of income.
DCEs are often based on rule of thumb approaches as they often include components that are not known prior to data analysis. One rule of thumb is that 20 respondents per questionnaire version [block] is sufficient for estimating reliable models, and over 100 respondents are able to provide a basis for modeling preference data.24 Another rule of thumb suggest that the sample size required for a DCE is a function of choice tasks, number of alternatives, and number of analysis cells (largest number of levels for any attribute), which is approximately 63 in this study.25 Therefore, our conservative estimated target sample size was n = 200–300, which was above the typical sample size of healthcare DCEs26 and in line with the expected recruitment feasibility in the Japanese online patient panel.
2.4 Statistical analysis
A conditional logit with dummy coding was used for the analysis of the DCE to estimate preference weights and RI. The preference weights for the attributes are presented in the same order as those in the draft vignettes. RI was calculated as a percentage based on the difference between the highest and lowest coefficient values. The distance between the highest and lowest coefficient values for each attribute was divided by the sum of the coefficient differences between the highest and lowest levels in all attributes and multiplied by 100. RI for attributes was subsequently ranked and presented from highest to lowest value for statistical interpretation. Sensitivity analysis excluding those who failed the dominance test was analyzed. Subgroup analyses were conducted for severity (moderate vs. severe), prior exposure to biologics (biologics-naïve vs. biologics experienced), residential area (urban vs. non-urban), presence of psoriatic arthritis (PsA) as a comorbid condition (yes vs. no), gender (male vs. female), age and employment status (employed vs. unemployed). Respondents who live in cities with a population of 500 000 or more were categorized as urban, in accordance with the Ministry of Health, Labour and Welfare in 2020.27 All data analyses were performed using SAS version 9.4.
The study was conducted according to the principles of the Declaration of Helsinki and approved by the Public Health Research Foundation (approval Number: 21I001).
3 RESULTS
3.1 Patient interview results
Patients spontaneously reported that effectiveness, costs, mode of administration, and safety as the most important treatment attributes, which was consistent with the draft vignettes of the DCE. Table S1 presents the concepts that patients spontaneously reported and example quotes for each concept. Most patients reported that the included attributes were relevant. Additionally, the majority of patients did not identify any attributes missing from the draft vignettes. After the review of patient responses and expert suggestions, some attributes were modified and finalized, and language used in the descriptions of attributes was updated for easier comprehension (Table 2).
| Attributes | Levels |
|---|---|
| Patients with a 75% or greater improvement in the extent or severity of psoriasis symptoms within 1 month of starting treatment |
5% (5 out of 100 patients) 10% (10 out of 100 patients) 30% (30 out of 100 patients) |
| Patients with a 75% or greater improvement in the extent or severity of psoriasis symptoms at 1 year after starting treatment |
45% (45 out of 100 patients) 65% (65 out of 100 patients) 95% (95 out of 100 patients) |
| Percentage of patients who develop infections that require hospitalization within 1 year of starting treatment (e.g., pneumonia or other infections that cause problems in the body) |
1% (1 out of 100 patients) 2% (2 out of 100 patients) |
| Percentage of patients who develop diarrhea during treatment (including patients who have frequent episodes) |
1% (1 out of 100 patients) 5% (5 out of 100 patients) 15% (15 out of 100 patients) |
| Administration mode and frequency |
Oral: Once daily Oral: Twice daily Hypodermic injection: Every 12 weeks |
| Monthly out-of-pocket medical expenses (reimbursed medication and administration cost) |
Monthly 18 000 JPY Monthly 19 000 JPY Monthly 28 000 JPY |
3.2 Demographics
Of the 222 patients who participated in the survey, 71% were male and mean age was 51 (Table 3); 86% of patients reported moderate and 14% reported severe psoriasis. Most patients (65%) were diagnosed with PsO more than 5 years prior, and about 24% of patients reported living with PsO for more than 20 years. At the time of survey completion, 83% of patients reported currently taking oral medications and 40% biologic therapies (Table S2). More than half of the patients (64%) were biologic-naïve prior to current therapy, and 48% lived in an urban area.
| Characteristics | n = 222 |
|---|---|
| Sex, n (%) | |
| Female | 64 (28.8) |
| Male | 158 (71.2) |
| Age, mean (SD), year | 50.9 (12.6) |
| Self-reported severity of PsO, n (%) | |
| Moderate | 191 (86.0) |
| Severe | 31 (14.0) |
| Time since PsO diagnosis, n (%) | |
| <1 year | 4 (1.8) |
| 1–2 years | 21 (9.5) |
| 2–5 years | 53 (23.9) |
| 5–10 years | 55 (24.8) |
| 10–20 years | 36 (16.2) |
| More than 20 years | 53 (23.9) |
| Current PsO treatment group (any), n (%) | |
| Any ointment | 185 (83.3) |
| Any phototherapy | 53 (23.9) |
| Any oral medicine | 184 (82.9) |
| Any biologics | 88 (39.6) |
| Previous PsO treatment, n (%) | |
| Biologic-naïve | 142 (64.0) |
| Biologic experienced | 80 (36.0) |
| Comorbid conditions, n (%) | |
| Type 2 diabetes | 40 (18.0) |
| Cardiovascular diseases | 14 (6.3) |
| Metabolic syndrome | 33 (14.9) |
| Psoriatic arthritis | 53 (23.9) |
| Malignancies | 3 (1.4) |
| None of the above | 105 (47.3) |
| Employment status, n (%) | |
| Employed (full-time and part-time) | 173 (77.9) |
| Unemployed | 49 (22.1) |
| Residential areaa | |
| Urban | 106 (47.8) |
| Non-urban | 116 (52.2) |
| Level of income, n (%) | |
| 11.6 million or more yen | 16 (7.2) |
| 7.7–11.6 million yen | 45 (20.3) |
| 3.7–7.7 million yen | 71 (32.0) |
| 1.5–3.7 million yen | 53 (23.9) |
| <1.5 million yen | 24 (10.8) |
| I prefer not to answer | 13 (5.9) |
- Abbreviations: PsO, psoriasis; SD, standard deviation.
- a Respondents were categorized into urban or non-urban groups depending on their residence. Urban residence was defined as cities designated by government ordinance from among cities with a population of 500 000 or more.27
3.3 Overall DCE results
Across the overall study population, long-term efficacy was the most important attribute (RI 47%), followed by cost (RI 21%) and mode of administration (RI 14%; Figure 2a). Risk of side effect (severe infections) was the least important compared to other attributes (RI 2%). The preference weights for long-term efficacy and costs were statistically significant, favoring higher long-term efficacy and lower costs (Figure 2b). Daily oral medications were preferred compared to hypodermic injection every 12 weeks (Figure 2b). Better safety outcomes were preferred with reference to gastrointestinal (GI)-related side effects while preferences related to severe infection side effects were only marginally different.
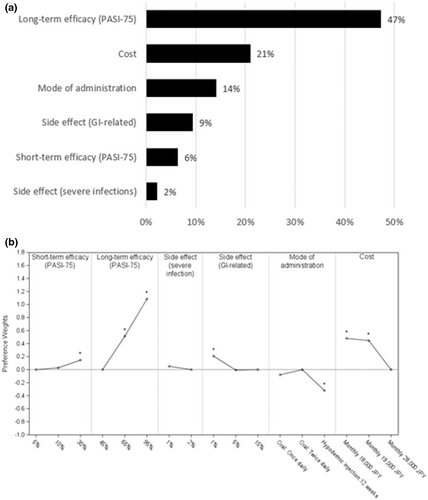
3.4 Sensitivity analysis
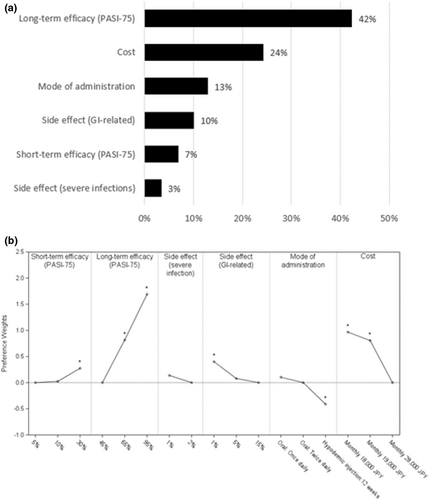
Sensitivity analysis was conducted excluding those who failed to select the dominant task in the rationality test. Excluding these patients did not change the overall results (Figure 3) in level of significance and direction of effect for most levels. Only the ranking of levels within mode of administration changed with “once daily” being numerically superior to “twice daily”, although there was no statistical difference.
3.5 Subgroup analyses
Long-term efficacy was consistently reported as the most important attribute across the subgroups. However, the second and third most important attributes differed among patients with different characteristics. Side effects (severe infection requiring hospitalization) was the least important attribute across all subgroups. Results from additional subgroup analyses are shown in Figures S1–S3.
3.6 Severity
Preferences differed between moderate and severe patients for most treatment attributes except long-term efficacy and side effects (severe infections). Regardless of severity, long-term efficacy was the most important attribute (Figure 4a). In severe patients, the second most important attribute was short-term efficacy (RI 21%), while in moderate patients, more emphasis was placed on costs (RI 21%), mode of administration (RI 16%), and GI-related side effects (RI 9%) over improving short-term efficacy (RI 4%). Moderate patients preferred oral medications over injections (Figure 4b). However, severe patients showed no significant difference in preference between oral medications and injections. Similarly, patients with moderate PsO preferred a treatment with lower risk of GI-related side effects, but patients with severe PsO did not show any difference. Furthermore, cost was an important attribute for moderate patients compared to severe patients. With lower cost, higher preference weight was observed among moderate patients. Severe patients had similar patterns of preference to the prior biologic exposed group and moderate patients with biologic-naïve group (Figure S2).
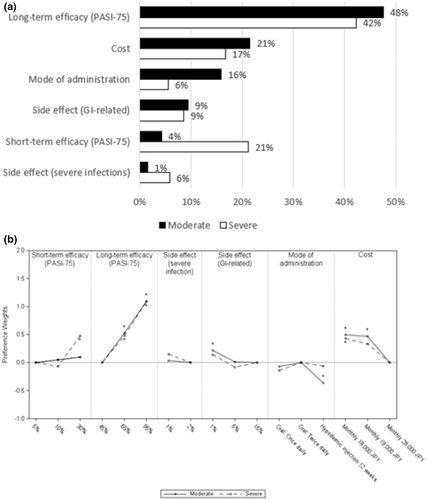
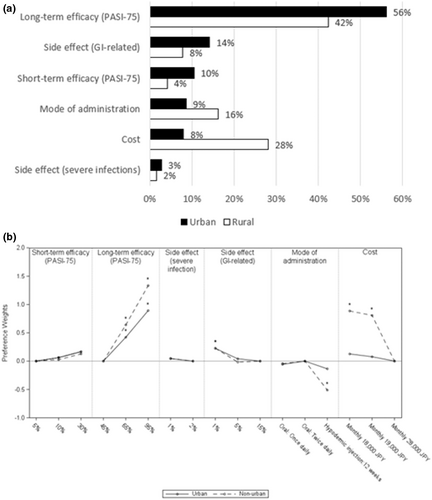
3.7 Residential area
Among patients living in non-urban areas, cost (RI 28%) was ranked as the second most important attribute followed by mode of administration (RI 16%); in contrast, the patients living in urban areas regarded GI-related side effects (RI 14%) as the most important attribute after long-term efficacy (RI 56%), followed by short-term efficacy (RI 10%; Figure 5a). Patients living in non-urban areas preferred oral medications over injections (Figure 5b). However, patients living in urban areas did not have any preference for mode of administration. Non-urban residents placed more importance on costs and had a preference for lower costs compared to urban residents.
3.8 PsA as a comorbidity
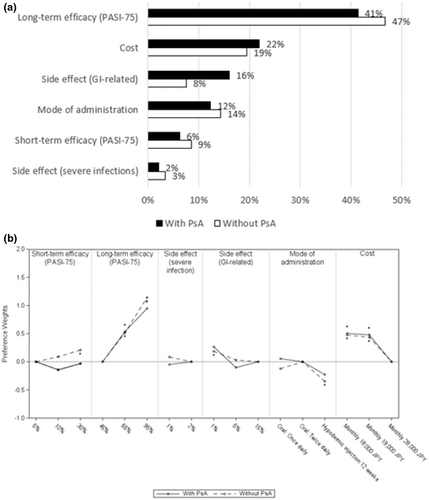
The most reported comorbidity among those surveyed was PsA (Table 3). Patients with PsA placed more importance on side effects (GI-related; RI 16%) than mode of administration (RI 12%; Figure 6a). In contrast, patients without PsA placed more importance on mode of administration (RI 14%) and short-term efficacy (RI 9%) compared to side effects (GI-related; RI 8%). Both patient groups, with and without PsA, preferred oral over injections, (p < 0.05 in non-PsA group; Figure 6b).
3.9 Gender
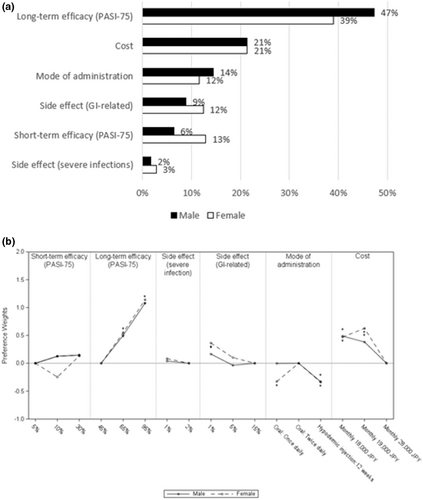
Long-term efficacy and cost were the most important attributes among both male and female patients. However male patients placed more importance on mode of administration (RI 14%) than on short-term efficacy (RI 6%) and side effects (GI-related; RI 9%), while women placed slightly more importance on short-term efficacy (RI 13%) and side effects (GI-related; RI 12.4%) than on mode of administration (RI 11.6%; Figure 7a). In terms of preference weights, oral medication was preferred compared to injection every 12 weeks among both males and females (Figure 7b). However, among females, taking a tablet once daily was less preferred compared to tablet twice daily.
4 DISCUSSION
This study used a DCE survey to investigate PsO treatment preferences among moderate-to-severe patients in Japan in terms of efficacy, safety, mode of administration, and costs. To develop the DCE attributes and levels, this study incorporated patient perspectives from qualitative interviews, in addition to the literature review and expert input.
Long-term efficacy was consistently reported as the most important attribute across the overall study population and subgroups. Sustaining long-term efficacy also remains a challenge in the real-world setting, particularly with biologics that are susceptible to anti-drug antibody formation.28 As patients with PsO frequently experience relapse of symptoms29 and have difficulty in controlling their symptoms over a long period, moderate-to-severe patients may prefer treatments with long lasting efficacy over short-term efficacy. Prior studies also reported that patients valued long-term efficacy more than rapid onset of treatment efficacy.21, 30
Mode of administration was, like efficacy and safety, relatively important across the overall study population and subgroups. Furthermore, patients preferred oral medication over injection, which was a consistent trend observed in other countries and therapeutic areas.18, 31, 32
Preferences varied across PsO severity (moderate vs. severe), with efficacy-related attributes being the most important among severe patients, while moderate patients placed greater value on mode of administration than short-term efficacy. This is consistent with other studies; patients with moderate PsO place more importance on mode of administration and preferred oral medications over injections.33
Preferences for treatment diverged for patients living in urban and non-urban areas, with patients living in non-urban areas preferring oral medication over injections as well as treatments with lower costs. In our study population, compared to urban areas, there was a higher proportion of patients living with PsO for more than 10 years in non-urban areas (Table S3). Economic burden of treatment for PsO may be substantial because patients often experience recurrence of symptoms, and patients with long-term PsO are likely to receive long-term treatment. In Japan, the yearly economic burden of biologics treatments was estimated to be 20 times higher than topical therapies.34 Given the potentially high economic burden, the higher proportion of those living with the disease for a long duration in non-urban areas may have contributed to the overall strong preference for lower costs. Further research that incorporates the socioeconomic factors, duration of PsO and how they impact treatment preference is needed to explore other underlying treatment needs.
Females placed more importance on short-term efficacy than other characteristics such as mode of administration and side effect (GI-related). Compared to male PsO patients, female patients tend to be more concerned about their appearance, a factor which was associated with self-reported depressive symptoms.35 Females reported itching36 and stigmatization37 more frequently than their male counterparts. These characteristics are associated with poorer quality of life and higher rates of depression among females.36, 38 Living with PsO may have a greater impact on female patients which could explain the preference for fast-acting treatments.
Side effect (severe infections) was the least important attribute for the overall population and subgroups, and there was no significant preference observed among levels. A lack of importance and clear preference may be due to the small difference between side effects levels (1% vs. 2%) for this attribute.
Several areas of further research could be considered. Results contrasted for preferences in different subgroups of severity, gender, and residential areas. More intensive exploration of other factors such as lifestyle differences, as well as analysis of other patient characteristics in these subgroups may provide more insight on patient preferences. Further research on the factor impacting treatment preferences for PsO is warranted.
4.1 Strengths and limitations
As the two previous Japanese PsO DCE studies only included physicians' inputs in the DCE development,20, 21 a strength of this study is that patient input on treatment preferences was integrated during the design phase. The results of the study highlighted the importance of long-term efficacy for PsO patients overall and in the analyzed subpopulations. We have also identified several relevant patient characteristics, including by severity, bio-experience, residence, comorbid PsA, and gender, that suggest differential preferences and RI for specific treatment attributes, especially in terms of cost, mode of administration, and short-term efficacy.
While this DCE study provided various insights into PsO patient preferences, several limitations should be noted. This DCE was limited to patients with self-reported moderate-to-severe PsO. If respondents' symptoms were well-controlled, they may not have been included in the study population because they perceived their condition to be milder, and therefore did not self-report as moderate-to-severe. However, since qualitative interviews incorporated patients who had a confirmed physician's diagnosis of moderate-to-severe PsO, the process of attribute development should accurately reflect their views.
Although the DCE survey may exclude moderate or severe patients who self-reported as mild, the extent of this potential misclassification cannot be quantified. Also, the bias was mitigated to some extent by including only patients with systemic pharmacotherapy as the final criterion; a criterion found to be clinically aligned for treating moderate-to-severe PsO patients in Japan.21, 39 In addition, about 25% of respondents failed the dominance test. This proportion of dominance test failure was similar to that reported elsewhere.40 However, the statistical significance of the preference weights in the sensitivity analysis was not different from the primary analysis results with the full sample. Lastly, the DCE's construct choice sets was based on hypothetical questions that may differ from real-world decision making. In an effort to minimize the gap between hypothetical and real-world choices, qualitative patient interviews were conducted to help develop of the attributes and levels in each choice set, reflecting patient experience and relevant attributes.
5 CONCLUSION
Treatment attribute preferences of moderate-to-severe patients receiving systemic PsO treatments were assessed after utilizing patient perspectives and narratives to guide the design of this study. This DCE employed the attributes of both oral and injection treatments and stratified preferences by disease severity and other patient characteristics. Acknowledging the high RI of long-term efficacy and preference for oral mode of administration, coupled with the differences in preference among subgroups will be important considerations when making treatment decisions for moderate-to-severe PsO patients.
CONFLICT OF INTEREST STATEMENT
MK has received grants or honoraria from Mebix, Leo pharma, Boehringer Ingelheim, Bristol Myers Squibb, Taiho Yakuhin, Kyowa Kirin, Abbvie, Torii Yakuhin, Tanabe Mitsubishi, Eisai, Sun Pharma, and Maruho. HK, KH and YH are employees of and report profit of stock or stock options from Bristol Myers Squibb. JY, YS and BC are employees of Syneos Health. SF reports Research, speaking, and/or consulting support from AbbVie, Accordant, Almirall, Alovtech, Amgen, Arcutis, Arena, Argenx, Biocon, Boehringer Ingelheim, Bristol Myers Squibb, Caremark, Celgene, Dermavant, Eli Lilly and Company, Eurofins, Forte, Galderma, GlaxoSmithKline/Stiefel, Helsinn, Informa, Janssen, Leo Pharma, Menlo, Merck & Co, Mylan, National Biological Corporation, National Psoriasis Foundation, Novartis, Novan, Ortho Dermatology, Pfizer, Qurient, Regeneron, Samsung, Sun Pharma, Sanofi, Teladoc, UCB, UpToDate, and vTv Therapeutics. Founder and part owner of Causa Research and holds stock in Sensal Health. This study was funded by Bristol Myers Squibb.
Open Research
DATA AVAILABILITY STATEMENT
Restrictions apply to the availability of these data, which were used under license for this study. The data that support the findings of this study are not publicly available and cannot be shared with external researchers.




