Crystal structure and substrate specificity of the β-ketoacyl-acyl carrier protein synthase III (FabH) from Staphylococcus aureus
Abstract
β-Ketoacyl-ACP synthase III (FabH), an essential enzyme for bacterial viability, catalyzes the initiation of fatty acid elongation by condensing malonyl-ACP with acetyl-CoA. We have determined the crystal structure of FabH from Staphylococcus aureus, a Gram-positive human pathogen, to 2 Å resolution. Although the overall structure of S. aureus FabH is similar to that of Escherichia coli FabH, the primer binding pocket in S. aureus FabH is significantly larger than that present in E. coli FabH. The structural differences, which agree with kinetic parameters, provide explanation for the observed varying substrate specificity for E. coli and S. aureus FabH. The rank order of activity of S. aureus FabH with various acyl-CoA primers was as follows: isobutyryl- > hexanoyl- > butyryl- > isovaleryl- >> acetyl-CoA. The availability of crystal structure may aid in designing potent, selective inhibitors of S. aureus FabH.
Bacterial β-ketoacyl-ACP synthase (KAS) enzymes are important in the elongation steps of fatty acid biosynthesis (Heath et al. 2001; Khandekar et al. 2003). KAS I (FabB) and KAS II (FabF) are involved in the condensation of malonyl-ACP with a growing acyl-ACP chain to form β-ketoacyl-ACP, which is a substrate for β-ketoacyl- ACP reductase (FabG). KAS III (FabH) catalyzes the initiation of fatty acid biosynthesis by condensing malonyl-ACP with acetyl-CoA. FabH from Gram-negative Escherichia coli has been studied extensively. It is encoded by the fabH gene, and is a homodimer with a monomeric molecular weight of 35 kDa (Han et al. 1998; Khandekar et al. 2003). It has been cloned, expressed, and purified by several groups, and has been extensively characterized both mechanistically and structurally (Qiu et al. 1999a; Davies et al. 2000; Khandekar et al. 2000). The E. coli FabH crystal structure has been solved in the presence and the absence of the substrate, acetyl-CoA (Qiu et al. 1999a, 2001; Davies et al. 2000). In the crystal structure, the close approximation of Cys112 to CoA suggests it may play an important role in catalysis. Modeling based on a bound CoA molecule has identified His244 and Asn274 as additional residues that might be involved in catalysis.
Staphylococcus aureus (S. aureus) is a Gram-positive human pathogen that causes diseases in humans, including skin infections, scalded-skin syndrome, and toxic shock syndrome. The bacterium is also the leading cause of nosocomial infections. S. aureus-induced disease is often suppurate and causes extensive tissue destruction and necrosis. Infections caused by S. aureus are often resistant to antibiotic treatments, and can reoccur even years after apparently successful therapy (Lowy 1998). There are few antibiotics on the market to combat S. aureus resistance and these are starting to fail; therefore, there is a growing need to develop new anti-bacterial agents against S. aureus.
Because they are essential enzymes for bacteria and differ significantly from human fatty acid synthase (FAS), various bacterial FabHs have been studied as potential anti-bacterial targets (Kaneda 1991; Han et al. 1998; Choi et al. 2000a,b; Davies et al. 2000; Khandekar et al. 2001; He and Reynolds 2002). Substrate specificity of the various FabH enzymes appears to be the determining factor in the biosynthesis of branched- or straight-chain fatty acids of the type II fatty acid synthase (Kaneda 1991; Choi et al. 2000a). Consistent with this notion, FabH purified from Gram-negative and Gram-positive bacteria, despite their overall similar catalytic mechanism, have displayed significantly different substrate specificities (Khandekar et al. 2003). For example, Streptococcus pneumonia, a Gram-positive bacterium, is able to utilize both straight- and branched-chain (C4–C6) acyl CoA primers (Khandekar et al. 2001), while E. coli, a Gram-negative organism FabH, utilizes primarily short straight-chain acyl CoAs—preferentially acetyl-CoA (Choi et al. 2000b).
To further understand the reasons for the observed differences in substrate specificities between Gram-negative and Gram-positive bacteria at a molecular level, we have determined the crystal structure of S. aureus FabH to 2.0 Å resolution. In this paper we show that similar to S. pneumoniae FabH, S. aureus FabH utilizes straight- and branched-chain (C4–C6) acyl CoA primers, and propose a structural basis for the observed primer specificity for the E. coli and S. aureus FabHs.
Results
Purification and characterization of S. aureus FabH
N-terminally HH-tagged S. aureus FabH was expressed and purified from E. coli cell pellets as described in Materials and Methods. The yield of S. aureus FabH following a Ni-NTA, Blue sepharose, and Superdex S 200 SEC was ∼3 mg/g. On SDS-PAGE, purified S. aureus FabH migrated as a 36-kDa polypeptide. By LC-MS, molecular weight of S. aureus FabH was determined to be 35,911 Da, as expected based on the primary amino acid sequence of the expressed protein. Similar to the reported studies (He and Reynolds 2002) S. aureus FabH in our studies migrated as a dimer on a size exclusion column (not shown).
For crystallography studies, purified S. aureus FabH was concentrated to 15 mg/mL in buffer containing 20 mM Tris (pH 8.0), 3 mM DTT, 100 mM NaCl, and 10% glycerol. 15 μL aliquot of concentrated protein was subjected to dynamic light scattering (Protein Solutions) and the data was deconvoluted using DynaPro software. The histogram (19% Cp/Rh, 0.04 Cp/Rh index, 4.387 Rh) showed only one peak (not shown), indicating monomodal size distribution.
Substrate specificity of FabH
The FabH-FabG spectrophotometric coupled assay was used to determine the substrate specificity of S. aureus and E. coli FabH for various acyl-CoA primers. Where radiolabeled acyl-CoA primers were available, these were used in the filter-binding assay to confirm the specificity of S. aureus FabH for these substrates (Table 1). In the coupled assay malonyl-ACP and acyl-CoA are condensed by S. aureus or E. coli FabH to yield β-ketoacyl-ACP, which is a substrate of S. pneumoniae FabG. FabG catalyzes the reduction of β-ketoacyl-ACP to β-hydroxyacyl-ACP and the concomitant oxidation of NADPH to NADP+, a coupled reaction which can be monitored spectrophotometrically at 340 nm. For S. aureus FabH, the highest activity was obtained with isobutyryl-, hexanoyl-, and butyryl-CoA (Table 1). In general, the rank order of activity of S. aureus FabH with various acyl-CoA primers was as follows: isobutyryl- > hexanoyl- > butyryl- > isovaleryl- >> acetyl-CoA (Table 1). In contrast, using this assay the highest activity for E. coli FabH was obtained using acetyl-CoA as primer (Table 1). E. coli FabH had the lowest activity when isobutyryl-, hexanoyl-, butyryl-, isovaleryl-, and lauroyl-CoA were used. These results are consistent with earlier studies (Choi et al. 2000b; Khandekar et al. 2001).
Kinetic characterization of S. aureus FabH
Kinetic parameters for acyl-CoA primers that showed the highest activity in S. aureus FabH assays were determined using the coupled assay format (Table 2). S. aureus FabH exhibited Michaelis-Menten kinetics at varied acyl-CoA concentrations and fixed malonyl-ACP concentration (not shown).
Apparent Km for malonyl-ACP was determined using isobutyryl-, hexanoyl-, butyryl-, and isovaleryl-CoA. For each, malonyl-ACP concentration was varied while the acyl-CoA concentration was fixed. At higher malonyl-ACP concentrations, weak substrate inhibition was observed; therefore, the data was fitted to Equation 1 and the results summarized in Table 3.
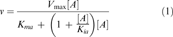 (1)
(1)E. coli FabH catalysis is thought to be carried out via a ping-pong mechanism when acetyl-CoA and malonyl-ACP are used (Jackowski and Rock 1987; Tsay et al. 1992a). In a ping-pong reaction mechanism one or more products are released before all the substrates are bound. Although the S. aureus FabH catalyzes the same condensation reaction, there is no data available to confirm the kinetic mechanism. Thus, the latter was determined by simultaneously varying the concentration of malonyl-ACP and isobutyryl-CoA, and the initial velocities were determined using the coupled assay. The data were fitted to Equation 2, a ping-pong model that takes into account substrate inhibition.
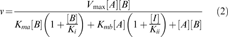 (2)
(2)A two-substrate kinetic analysis of malonyl-ACP condensation with isobutyryl-CoA by S. aureus FabH suggests that the reaction proceeds by a ping-pong kinetic mechanism (not shown).
Diffraction data collection and structure solution
The S. aureus FabH crystals belong to the monoclinic spacegroup P21, with unit cell dimensions a=63.9 Å, b=93.6 Å, c=110.0 Å, and d=93.9°. The diffraction data, collected at the IMCA-CAT of Advanced Photon Source of Argonne National Laboratory, are 96.6% complete to 2.0 Å resolution with an overall redundancy of 3.0, mosaicity of 0.3°, I/σ of 12.6 (last shell 2.6), and Rmerge of 0.085 (last shell 0.23). There are four FabH molecules (two dimers) per asymmetric unit and a solvent content of about 50%. The structure was solved with the molecular replacement programs in the CCP4 suite (Collaborative Computational Project 1994) using an E. coli FabH monomer structure (Qiu et al. 2001) as the search model. The correct solution gave an R-factor of 0.47 after rigid body refinement to 4 Å resolution. Solvent flattening, histogram matching and twofold averaging were used to extend the electron density map to 2.0 Å resolution. Iterative manual model building and CNS (Brunger et al. 1998) refinement using all data from 20.0 to 2.0 Å resolution gave an R-factor of 0.211 (last shell 0.259) and an Rfree of 0.256 (last shell 0.301). All 1248 (4 × 312) residues are observed, including 792 water molecules. Pro88 and Leu302 are found to be cis-peptides. The final rms values are 0.011 Å for bond length and 1.5° for angles; 89.2% of the residues fall in the most favorable region of the Ramachandran plot, with 0.7% in the generously allowed regions and none in the disallowed regions.
Overall structure of S. aureus FabH
The structures of two S. aureus FabH dimers have been determined independently in the crystal asymmetric unit. They have similar overall structures and can be superimposed well. The four independently determined S. aureus FabH monomers are nearly the same (RMS Cα between molecules: A–B, 0.35 Å; A–C, 0.24Å; A–D, 0.43 Å; and B–D, 0.28 Å). The overall structure of S. aureus FabH is also similar to that of E. coli FabH (RMS Cα of 1.0 Å), but with differences in insertion (1 residue in the 169–173 loop and 1 residue at the C terminus) and deletion (7 residues in the 193–195 loop) regions (Fig. 1). Most notably, the S. aureus FabH is 7 residues shorter than E. coli FabH in the 193–195 loop that flanks FabH dimer interfaces (Fig. 1). While this observation seems to suggest differences in S. aureus FabH and E. coli FabH dimer stability, it is probably insignificant due to the known flexibilities in this part of the dimer interface based on our previous studies (Qiu et al. 2001).
Catalytic residues
As in E. coli FabH (Qiu et al. 2001), the catalytic residues of S. aureus FabH include Cys112, His238 (E. coli His244) and Asn268 (E. coli Asn274). Most of the side chains have similar conformations for residues lining the active sites in the four independently determined S. aureus FabH monomers. However, the Phe298 (E. coli Phe304) side chain can rotate about 150° in the various FabH structures (Fig. 2). These structures suggest that Phe298 can form aromatic stacking interactions with His238 (distance ∼3.6 Å) in the absence of substrates to limit solvent exposure of the catalytic residues. In the presence of substrate, the observed flexibility will push the Phe298 side chain to swing away from His238 and possibly guide and allow substrate entrance.
Primer binding site
The acyl primer binding pocket of S. aureus FabH can be effectively defined by superposition with the known E. coli FabH primer binding pocket (Qiu et al. 2001) at Cys112. As shown in Figure 3, the E. coli FabH binding pocket, large enough to accommodate acetyl- or propionyl- CoA, is notably smaller than that of S. aureus FabH. In S. aureus FabH, side chains shift concertedly away from Cys112 to create additional room for larger primers, even though the amino acid sequences are identical for these residues in E. coli FabH and S. aureus FabH. Some of these observed shifts are small, e.g., Leu190 (E. coli Leu189), Leu199 (E. coli Leu205), and Phe157 (E. coli Phe157) that moved 0.5, 0.8 and 1.1 Å, respectively. On the other hand, the Phe87B side chain of the other monomer moved by 1.7 Å, while the Leu142 side chain shifted by as much as 3.0 Å. The Phe87B shift was made possible by sequence differences at the dimer interface (i.e., the 193–195 loop) that allowed the adjacent Leu192 (E. coli Leu191) to move back 2.3 Å. The Leu142 difference is due to the large sequence difference among residues 141–146 (KLSKIT in S. aureus FabH vs. VLARTC in E. coli FabH). Therefore, even though the residues in direct contact with acyl primers have identical amino acid sequences for S. aureus FabH and E. coli FabH, these second shell sequence differences enable the observed increase in primer binding pocket size in S. aureus FabH.
Discussion
Fatty acid synthase enzymes in bacteria differ significantly from their eukaryotic counterparts. Hence, they have been studied extensively as potential targets for the discovery of novel anti-bacterial agents. S. aureus has cytoplasmic membranes which are composed of approximately 80% branched-chain fatty acids, and thus requires an efficient system by which branched-chain primers can be incorporated (Choi et al. 2000b). In this study, we examined the substrate specificity of S. aureus FabH and its ability to use acyl-CoA primers other then acetyl-CoA. Our results indicate that S. aureus FabH can utilize isobutyryl-, hexanoyl-, butyryl-CoA and also to some extent isovaleryl- and propionyl-CoA as substrates. Acetyl-CoA, on the other hand, was a poor substrate for S. aureus FabH. The substrate specificity of S. aureus FabH is consistent with previous data on the incorporation of branched-chain primers into cellular fatty acids (Kaneda 1963; Choi et al. 2000b). FabH of S. glaucescens, which synthesizes branched-chain fatty acids, has also been shown to use isobutyryl- and butyryl-CoA as substrates (Han et al. 1998). Thus, the FabH proteins from organisms that synthesize branched-chain fatty acids exhibit broader substrate specificity than those from organisms that synthesize straight-chain fatty acids, which are highly selective and specific for acetyl-CoA. The overall order of substrate specificities of various acyl CoAs for S. aureus FabH were similar to those reported earlier (He and Reynolds 2002); however, the Km and Kcat reported by them for the various acyl CoAs were significantly different. In our studies, the Km values for isobutyryl CoA, butyryl CoA, and acetyl CoA were determined to be 37 μM, 20 μM, and 373 μM, respectively. The Km values reported by He and Reynolds for isobutyryl CoA, butyryl CoA, and acetyl CoA were 0.32 μM, 2.32 μM, and 6.18 μM, respectively. Likewise, the Kcat values were 10– 30 times higher than those reported previously (He and Reynolds 2002). Since the purity and biochemical properties S. aureus FabH used in our studies were comparable in these two studies the most likely reason for these discrepancies is the differences in the assay conditions. While He and Reynolds used a radioactive filter binding assay, we used a S. aureus FabH-S. pneumoniae FabG-coupled assay. It is possible that although the affinity of S. aureus FabH for these acyl CoAs is high, the affinity of S. pneumonia FabG for acyl-ACP might be low, thus yielding a lower rate, which in turn would result in higher Km. Further work will help explain these differences.
The substrate specificity of FabH from different species can be attributed to the differences in its structure from these species. The acetyl methyl group takes up most of the space of the primer binding site in the E. coli FabH structure (Qiu et al. 2001). In contrast, the S. aureus FabH primer binding pocket is larger (Fig. 3A) and is able to accommodate acyl-CoA primers that are larger than acetyl-CoA. Specifically, the Phe87B and Leu142 shifts open up the primer binding pocket, allowing acyl primers such as isobutyryl to fit in, as modeled in Figure 3B. This is consistent with results from our enzymatic study, which shows that unlike E. coli FabH that prefers acetyl-CoA as a substrate (Table 1), S. aureus FabH has evolved to use long straight- and branched chain acyl-CoA primers (Table 1).
The larger binding pocket of S. aureus FabH can accommodate both straight- and branched-chain acyl-CoA primers; however, there appears to be an optimum size for the acyl-CoA primers that can fit into this binding pocket of S. aureus FabH. The C4-C6 acyl-CoA primers are likely to fit better in the S. aureus FabH binding pocket, which results in increased enzyme activity. Likewise, anything smaller than a C4 acyl-CoA is a poor substrate for S. aureus FabH. The kinetic parameters confirm the preference of S. aureus FabH for C4-C6 acyl-CoA primers as shown in Table 2. Not surprisingly, the affinity of S. aureus FabH for malonyl-ACP remains relatively constant regardless of the acyl-CoA primer used (Table 3).
Interestingly, the FabH homolog in Mycobacterium tuberculosis catalyzes extension of much longer acyl chains (lauroyl, myristoyl, and palmitoyl) for cell wall mycolic acid synthesis (Choi et al. 2000b). As expected, the crystal structure of wild-type M. tuberculosis FabH (Scarsdale et al. 2001) and its C112A mutant in complex with lauroyl-CoA (Musayev et al. 2005) revealed an elongated channel extending from the catalytic cysteine that is significantly larger than those of E. coli FabH and S. aureus FabH. In M. tuberculosis FabH, the F87′ equivalent of E. coli FabH is a threonine, thus permitting the formation of a binding site for longer acyl chains (Scarsdale et al. 2001). While the overall sequences and structures of these FabH enzymes are quite similar, the seemingly small sequence differences at the dimer interface allow the formation of enzymes with notably different substrate specificities.
Isobutyryl-CoA was used in the coupled assay to determine the mechanism of S. aureus FabH. The data obtained by varying isobutyryl-CoA and malonyl-ACP concentrations best fit a ping-pong equation. For E. coli FabH it has been shown that acetyl-CoA binds first to the enzyme followed by the release of CoA-SH. Malonyl-ACP then binds to the acetylated enzyme and the condensation reaction occurs followed by the release of the products CO2 and β-ketoacyl-ACP (Jackowski and Rock 1987; Tsay et al. 1992a). It is highly likely that S. aureus FabH will exhibit the same mechanism.
Bacterial type II FAS system has been studied as a source of anti-bacterial targets, and has considerable potential for selective inhibition by broad-spectrum anti-bacterial agents. For example, triclosan inhibits bacterial FabI (McMurry et al. 1998; Qiu et al. 1999b); the anti-tuberculosis drug isoniazid inhibits enoyl-ACP reductase and potentially other components of mycolic acid synthesis (Banerjee et al. 1994; Mdluli et al. 1998; Slayden et al. 2000). E. coli FabB and FabF are inhibited by cerulenin (Edwards et al. 1997; Price et al. 2001) and thiolactomycin (TLM) (Jackowski et al. 1989; Price et al. 2001), whereas the human FAS is inhibited by cerulenin but not TLM (Khandekar et al. 2001). E. coli FabH is not inhibited by cerulenin and is only weakly inhibited by TLM(Tsay et al. 1992b). This is understandable because cerulenin binding relies heavily on interactions in the primer binding site, which is relatively small in most FabH species, especially E. coli FabH. Our study demonstrates that the primer binding site structure in S. aureus FabH is different from that of E. coli FabH, in spite of the identical sequence for all amino acids in direct contact with acyl primers. Based on the observed substrate specificity and structural arrangement in the active site, E. coli FabH inhibitor potency most likely comes from binding interactions to the catalytic residues as well as the CoA binding tunnel (Qiu et al. 2001). It will be interesting to find out whether the primer binding site can contribute to inhibitor design, especially for FabHs with larger pocket such as S. aureus FabH.
Materials and methods
Acyl-CoA primers and NADPH used in this study were purchased from Sigma. [1-3H]Acetyl-CoA and [1-14C]butyryl-CoA were from Moravek. Cloning and expression reagents were from Qiagen. Plasmid pET28a+ was from Novagen. E. coli FabH was purified as described previously (Khandekar et al. 2000). S. pneumoniae FabG was expressed and purified from E. coli cell lysate (T. Choudhry and J. Lonsdale, unpubl.). All other reagents were ACS grade or better.
Expression of S. aureus FabH
A plasmid directing the overproduction of S. aureus FabH with a (His)6 tag (hexahistidine (HH) tag) followed by a thrombin (LVPRGS) cleavage site at its N terminus was constructed similar to that described for S. pneumoniae FabH (Khandekar et al. 2001).
Purification of S. aureus FabH
Twenty grams of E. coli cells over expressing tagged S. aureus FabH were resuspended in 200 mL of lysis buffer (buffer A) containing 50 mM Tris-HCl (pH 8.0), 300 mM sodium chloride, 0.2 mM PMSF, 3 mM 2-mercaptoethanol, and 10% glycerol. Cells were lysed twice at 10,000 psi using Microfluidizer (Microfluidics Corp.). Cell debris was removed by centrifugation (Sorvall RC-5B) at 30,000g for 30 min. The supernatant was first batch-bound to a 45-ml Ni-NTA metal affinity column (Qiagen) for 2 h. The material was then transferred to a column and the column was washed with 10 column volumes of each buffer A and buffer A containing 40 mM imidazole at 5 mL/min. The bound material was eluted with buffer A containing 300 mM imidazole. FabH fractions were dialyzed against buffer B containing 20 mM Tris-HCl (pH 8.0), 100 mM NaCl, 10% glycerol, 3 mM DTT, and 3 mM EDTA, and next applied to a 25-mL blue sepharose column (Pharmacia) equilibrated in buffer B. After washing, the bound material was eluted with a five-column volume linear gradient of 0–1.0 M sodium chloride in buffer B. FabH, which eluted at 1 M NaCl, was next applied to a Superdex 200 size-exclusion column (2.6 × 60 cm, Pharmacia) equilibrated in 20 mM Tris-HCl (pH 7.5), 100 mM NaCl, 10% glycerol and 3 mM DTT.
Analytical studies
LC/MS analysis on purified preparations of S. aureus FabH were carried out as described (Feng et al. 2002). Dynamic light scattering (DLS) analysis was performed on purified S. aureus FabH (15 mg/mL) using a DynaPro-MSTC instrument (Protein Solutions, Inc.) as described previously (Khandekar et al. 2001).
Spectrophotometric assay
A continuous assay format was used to monitor S. aureus FabH activity with straight- and branched-chain acyl-CoA primers by coupling the condensing activity of S. aureus or E. coli FabH to β-ketoacyl- ACP reductase (FabG) of S. pneumoniae. S. aureus and E. coli FabH were routinely assayed using a reaction mixture consisting of 0.1 M sodium phosphate buffer (pH 7.0), containing 0.01% CHAPS, 7 μM malonyl-ACP, 100 μM NADPH, 1.5 μg S. pneumoniae FabG, 50 μM acyl-CoA, and 1 mM TCEP. It was aliquoted into 96-well half-area plates (Costar) to a final volume of 150 μL and incubated at 30°C for 5 min. The reaction was initiated by adding either 0.01 μg S. aureus FabH or 0.005 μg E. coli FabH to the wells, mixed, and the plate read at 340 nm using a SpectraMax Plus (Molecular Devices) plate reader.
S. aureus FabH kinetic parameters
S. aureus FabH kinetic parameters for acyl-CoA and malonyl-ACP were determined using the spectrophotometric assay format described above. A reaction mixture containing 30 μM malonyl-ACP, 5–300 μM acyl-CoA, 100 μM NADPH, 1.5 μg S. pneumoniae FabG, and 0.01 μg S. aureus FabH was prepared. It was aliquoted in 96-well half-area plates and incubated at 30°C. The reaction was initiated with S. aureus FabH and the plate read at 340 nm using a SpectraMax Plus. To determine Km for malonyl-ACP using isobutyryl-, hexanoyl-, butyryl-, and isovaleryl-CoA, the reaction conditions were the same as those described above except that the concentration of the acyl-CoA was 70 μM and the concentrations of malonyl-ACP ranged from 2–60 μM. For both experiments the data was fitted to the Michaelis-Menten equation to generate apparent Km values. The continuous coupled assay was also used to determine the kinetic mechanism of S. aureus FabH, the concentrations of isobutyryl-CoA and malonyl-ACP were varied from 10– 300 μM and 5–80 μM, respectively. The concentrations of NADPH, S. pneumoniae FabG, and S. aureus FabH were the same as indicated above.
Radiometric assay
A modified filter-binding assay, as described by Tsay and coworkers (Tsay et al. 1992a), was used to assay the activity of S. aureus FabH with acetyl-CoA and butyryl-CoA (He and Reynolds 2002). The assay contained 50 μM [3H]acetyl-CoA (specific activity 5.2 Ci/mmol) or [14C]butyryl-CoA (specific activity 54 mCi/mmol), 10 μM malonyl-ACP, and either S. aureus FabH (0.5 μg) or E. coli FabH (0.002 μg) in 0.1 M sodium phosphate buffer (pH 7.0), containing 0.01% CHAPS in a final volume of 150 μLa. The reaction was initiated by the addition of S. aureus FabH, and the reaction incubated at 30°C for 20 min. To stop the reaction, 10% trichloroacetic acid (TCA) was added and the reaction mixture incubated on ice for 30 min prior to filtration. Reaction mixtures were filtered and washed with 10% TCA on Packard GF/C filter plates using a Packard Filtermate harvester. The filter plates were dried and the radio-activity quantified using Wallac Supermix scintillation mixture and a Wallac Microbeta 1450 liquid scintillation counter.
Crystallization of S. aureus FabH
For crystallization, 2 μL of purified S. aureus FabH protein at 15 mg/mL in 20 mM Tris (pH 8.0), 100 mM NaCl, 3 mM DTT, and 10% glycerol was mixed with 2 μL of reservoir solutions (Hampton) at various conditions. The diffraction quality crystals of FabH grew from sitting drops equilibrated through the vapor phase at room temperature with a reservoir of 500 μL of solution containing 30% PEG 4000, 0.1 M Na citrate (pH 5.6), and 0.2 M ammonium acetate in a week.
Accession number
The atomic coordinates for the protein structure have been deposited into the Brookhaven Protein Data Bank under the accession number 1ZOW.
| Acyl-CoA | S. aureus FabH activity (pmol/min/ng) | E. coli FabH activity (pmol/min/ng) |
|---|---|---|
| Isobutyryl-CoA (4:0) | 31.4 ± 1.4 | 5.3 ± 0.8 |
| Hexanoyl-CoA (6:0) | 23.8 ± 0.5 | 3.4 ± 0.6 |
| Butyryl-CoA (4:0) | 21.7 ± 0.4 | 5.3 ± 0.2 |
| [14C]butyryl-CoA (4:0) | 19.4 ± 1.8 | n.d. |
| Isovaleryl-CoA (5:0) | 14.9 ± 0.5 | 3.8 ± 0.2 |
| Propionyl-CoA (3:0) | 12.5 ± 0.6 | n.d. |
| β-Methylcrotonyl-CoA (5:1) | 6.0 ± 1.8 | n.d. |
| Palmitoyl-CoA (16:0) | 5.0 ± 0.9 | n.d. |
| Lauroyl-CoA (12:0) | 3.4 ± 0.4 | 3.3 ± 0.2 |
| Acetyl-CoA (2:0) | 1.0 ± 0.1 | 50.5 ± 4.5 |
| [3H]acetyl-CoA (2:0) | 0.6 ± 0.05 | 46.8 ± 3.4 |
- a Enzyme activity was determined using 50 μM acyl-CoA, 7 μM malonyl- ACP, 100 μM NADPH, 1.5 μg S. pneumoniae FabG and 10.0 ng S. aureus FabH or 5 ng of E. coli FabH. Assay conditions were as described in Materials and Methods. n.d., not determined.
| Acyl-CoA | Km (acyl-CoA) (μM) | kcat (min−1) | kcat /Km (M−1min−1 × 106) |
|---|---|---|---|
| Isobutyryl-CoA | 37 ± 6 | 1682 ± 43 | 45 |
| Hexanoyl-CoA | 13 ± 2 | 1528 ± 84 | 118 |
| Butyryl-CoA | 20 ± 2 | 1254 ± 48 | 63 |
| Isovaleryl-CoA | 13 ± 2 | 930 ± 47 | 72 |
| Acetyl-CoA | 373 ± 17 | 261 ± 34 | 1 |
- a Substrate concentrations for apparent Km determinations for S. aureus FabH were as follows: 30 μM malonyl-ACP,5–300 μM acyl-CoA, 100 μM NADPH, 1.5 μg S. pneumoniae FabG, and 0.01 μg S. aureus FabH.
| Acyl-CoA | Km (malonyl-ACP) (μM) | kcat (min−1) | kcat /Km (M−1 min−1 × 106) |
|---|---|---|---|
| Isobutyryl-CoA | 24 ± 6 | 2827 ± 425 | 118 |
| Hexanoyl-CoA | 21 ± 7 | 2500 ± 589 | 119 |
| Butyryl-CoA | 22 ± 10 | 2498 ± 772 | 114 |
| Isovaleryl-CoA | 14 ± 7 | 1832 ± 594 | 130 |
| Acetyl-CoA | 19 ± 7 | 431 ± 113 | 23 |
- a Substrate concentrations for apparent Km determinations for S. aureus FabH were as follows: 70 μM acyl-CoA, 2–70 μM malonyl-ACP, 100 μM NADPH, 1.5 μg S. pneumoniae FabG, and 0.01 μg S. aureus FabH.
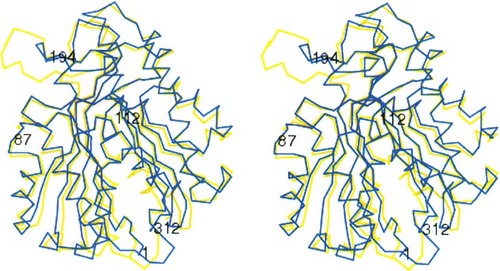
Stereo view of the overlay of the Cα structures of S. aureus FabH (blue) and E. coli FabH (yellow) monomers. N and C termini are labeled as 1 and 312. 112 denotes the catalytic residue Cys112. The substrates enter through a tunnel at the upper right corner between helices, while the other monomer of the FabH dimer fits at the left side (Qiu et al. 2001).
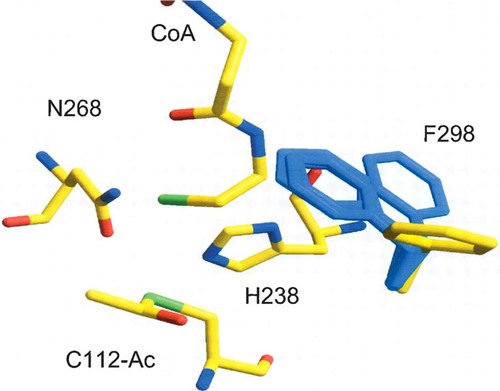
The conformation of Phe298 of S. aureus FabH (blue) in the overlay with the acetylated-E. coli FabH structure (yellow). The catalytic residues are Cys112, His238, and Asn268 in S. aureus FabH. The bound coenzyme A is labeled as CoA.
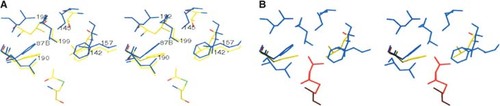
Stereo view of the primer binding sites in S. aureus FabH (blue) and E. coli FabH (yellow). (A) Acetylated Cys112 of E. coli FabH is shown at the bottom of the picture. S. aureus FabH residue numbers are labeled. 87B denotes Phe87 from the other monomer of the FabH dimer. (B) Model of isobutyryl (red) in the primer binding site in the same view. Only Phe87B and Leu142 of E. coli FabH are shown (yellow).




