Treatment of Refractory Pediatric Autoimmune Hepatitis With Rituximab
The authors report no conflicts of interest.
INTRODUCTION
Autoimmune hepatitis (AIH) is a progressive inflammatory liver disorder that is characterized by elevated aminotransferases, hypergammaglobulinemia, presence of autoantibodies, and histologic evidence of interface hepatitis (1, 2). Given the chronic nature of AIH, patients who are diagnosed late, do not achieve remission, or have relapsing courses can progress to cirrhosis and end-stage liver disease requiring liver transplantation (3). To decrease hepatic injury, the definition of remission has evolved to include not just normalization of aminotransferases but also reduction in immunoglobulin and autoantibody levels, and resolution of histologic inflammation (1, 2).
Immunosuppression with steroids and azathioprine induces biochemical remission in 80%–90% of patients (1). Treatment of refractory disease includes cyclosporine, mycophenolate mofetil, tacrolimus, sirolimus, infliximab, or rituximab (1, 2). Although AIH is predominantly a T cell-mediated process, mounting evidence also supports a key role for B cells (4). Successful treatment of refractory AIH can be achieved with rituximab, a monoclonal antibody against the B-cell surface protein CD20 (5-7), and B-cell depletion with ianalumab which is currently being studied in adults (2). Experience with rituximab in pediatrics is limited to 1 report of 2 patients (8) and dosing frequency is not standardized in adults or children (5-8). We report our experience using rituximab to successfully treat refractory AIH in 2 additional pediatric patients.
CASE 1
A 5-year-old woman with morphea and vitiligo presented with elevated aminotransferases, jaundice, and fever. Antinuclear and liver–kidney microsomal antibodies were elevated with titers of 1:5120, and >1:10 240, respectively. Liver biopsy revealed active chronic hepatitis with patchy bridging necrosis consistent with AIH type 2 (Fig. 1A). She was started on prednisone and azathioprine and achieved clinical remission. Over the next 2 years, she alternated between azathioprine for AIH and mycophenolate mofetil for adjunctive morphea treatment due to intercurrent flares. Two years after diagnosis while on mycophenolate mofetil, a liver biopsy was performed for the persistent elevation of her aminotransferases and revealed interface hepatitis and giant cell hepatocyte transformation consistent with active AIH with grade II, stage II inflammation. Despite 6-weeks of prednisone 1 mg/kg/day, repeat liver biopsy showed AIH grade I, stage II. Given her refractory disease, we treated her with rituximab 375 mg/m2 followed by 1 g/kg IVIG (Fig. 1). While immunoglobulin levels could not be followed as a marker of remission due to IVIG administration, she demonstrated good response with near normalization of her aminotransferases (Fig. 1A). Furthermore, as dramatic reduction in aminotransferases continued despite B-cell repletion, repeat rituximab doses were administered based on laboratory trends (Fig. 2). After remission, she continues to receive rituximab and IVIG infusions every 6–8 months based on aminotransferases and CD19 counts (Fig. 1B) and has transitioned from mycophenolate mofetil to methotrexate for morphea control. Since starting rituximab 5 years ago, her AIH has remained well-controlled.
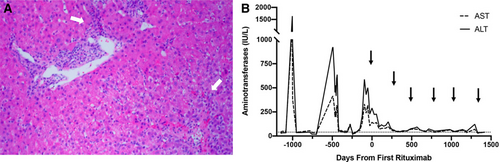
Histology and laboratory trends for patient 1. A) Liver histology with hematoxylin and eosin stain (10x magnification) was obtained during flare, just before rituximab initiation, and demonstrated patchy areas of chronic hepatitis (white arrows) with mild inflammatory activity centered on portal tracts and extending to the parenchyma. There was also mild portal fibrosis with few bridges (grade 1, stage 2). B) Aminotransferase levels improved from the time of first rituximab dose up to 4 years follow-up with all rituximab doses indicated by black arrows. Dotted line represents aminotransferase level of 40 IU/L. ALT = alanine aminotransferase; AST = aspartate aminotransferase.
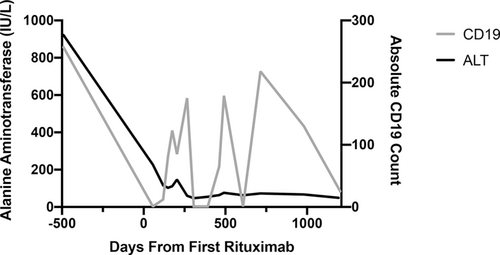
Correlation of CD19+ B-cell depletion and improvement in alanine aminotransferase (ALT) in patient 1 over 4-year follow-up.
CASE 2
An 18-year-old woman diagnosed with steroid-dependent AIH and arthritis in 2015 transferred care to our center in 2018. She was initially started on high-dose prednisone and azathioprine with improvement in aminotransferases although she never achieved biochemical remission. Mycophenolate mofetil was added as an adjunctive agent, but she had recurrent admissions for disease flares despite treatment with 3 immunosuppressive medications. Given her refractory disease, azathioprine was discontinued, and tacrolimus was started with brief normalization of laboratory parameters before another disease flare requiring high-dose steroids. Liver biopsy demonstrated chronic hepatitis with prominent portal and periportal fibrosis consistent with grade IV, stage III AIH and she was switched to sirolimus as rescue therapy (Fig. 3A). Prednisone was dosed following adult guidelines (patient weight > 60 kg). However, she was unable to wean below 20 mg daily due to persistently elevated aminotransferases, so she was treated with rituximab 375 mg/m2 followed by 1 g/kg IVIG. She received four rituximab and IVIG infusions over the first year and while immunoglobulin levels could not be followed for assessment of remission, her aminotransferases markedly improved (Fig. 3B) and she exhibited improved energy levels and reduced joint pain. At follow-up, she remains on maintenance with rituximab, adjunctive sirolimus, and low-dose prednisone (7.5 mg daily) and has not required admission for a disease flare in the year and a half since starting rituximab.
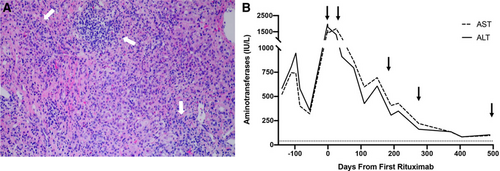
Laboratory trends and histology for patient 2. A) Liver histology with hematoxylin and eosin stain (10x magnification) was obtained during flare, just before rituximab initiation, and demonstrated chronic hepatitis (white arrows) with severe inflammatory activity involving the portal tracts and extending into the parenchyma. There were areas of parenchymal collapse, fibrosis, bridges, and occasional parenchymal nodules (grade 4, stage 3). B) Laboratory trend of aminotransferases showed improvement in levels from the time of first rituximab dose up to 500 days follow-up with all rituximab doses indicated by black arrows. Dotted line represents aminotransferase level of 40 IU/L. ALT = alanine aminotransferase; AST = aspartate aminotransferase.
DISCUSSION
We describe 2 pediatric patients successfully treated with rituximab for refractory AIH that demonstrated significant improvement although incomplete biochemical remission. Our data add to the mounting evidence of the important role that B cells play in the pathogenesis and treatment of AIH. Although some autoantibody titers and hypergammaglobulinemia correlate with disease activity, not all autoantibodies play a pathogenic role in AIH (1-4). In addition to antibody production, B cells serve as antigen-presenting cells that regulate T cells in AIH as supported by murine models of B-cell depletion that resulted in diminished numbers and cytotoxicity of CD8+ T cells (4). Furthermore, murine models of AIH demonstrate a role for B cell-driven cytokine production, including secretion of the proinflammatory cytokines IFN-γ and TNF-α as well as decreased secretion of the anti-inflammatory cytokine IL-10 (4). Finally, the chemokine CXCL10 involved in lymphocyte recruitment is diminished after AIH treatment with rituximab (4), providing another mechanism in which B cells may be critical to the regulation of the immune response in AIH.
Overall, these studies and our case series support the efficacy of B-cell depletion as an evolving salvage therapy of recalcitrant AIH in pediatrics. Given that 10%–20% of patients are refractory to standard therapies and 10%–15% will develop end-stage liver disease and require transplantation (9), having alternative treatment modalities for pediatric patients is critical. Furthermore, 14%–44% of patients with AIH have additional autoimmune comorbidities (2) similar to the patients in our report. These patients with more systemic involvement may represent a more difficult-to-treat subgroup in need of B-cell depleting therapies in addition to standard AIH treatment regimens. Although rituximab has not yet been endorsed as second-line therapy in AIH (2), we demonstrate efficacy without adverse events with dosing interval based on laboratory parameters. Further studies are needed to define the efficacy of B-cell modulation in the treatment of AIH and ultimately develop new treatment strategies to improve patient outcomes.




