Presentation and Outcomes of Infants With Idiopathic Cholestasis
A Multicenter Prospective Study
URL: https://childrennetwork.org/Clinical-Studies ClinicalTrials.gov Identifier: NCT00061828.
This study is funded by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (U01DK103149 to P.H., M.F., and B.S.; U01DK062466 and UL1TR001857 to J.S.; U01DK062470 and UL1TR002378 to N.G.; U01DK062500 and UL1TR001872 to L.B. and P.R.; U01DK084575 and UL1TR002319 to K.M.; U01DK084538 and UL1TR000130 to N.K.; U01DK103135 to V.N.; U01DK084536 and UL1TR001108 to J.M.; U01DK062497 and UL1TR000077 to J.B.; U01DK062481 and UL1TR001878 to K.L.; U01DK062436 and UL1TR000150 to S.T.; U01DK062503 and UL1TR000448 to K.S.; U01DK062452 and UL1TR000448 to Y.T.; U24DK062456 to J.M.; U01DK062453 and UL1TR002535 to R.S.).
P.R. reports consulting for Abbvie, Gilead, Retrophin, Albireo, Mirum, Audentes, Dicerna, Ambys; and grant support from Abbvie, Gilead, Merck, Retrophin, Albireo, Mirum, Arrowhead. K.M.L. reports grant support and consulting for Albireo Pharma and Mirum Pharmaceuticals. J.P.M. reports support from Abbvie, Gillead, Albireo Pharma, Mirum Pharmaceuticals, and CF Foundation. N.K. is on the Advisory Panel for High Tide. For the remaining authors, none is declared.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's Web site (www.jpgn.org).
ABSTRACT
Objectives:
The aim of the study was to determine the frequency and natural history of infantile idiopathic cholestasis (IC) in a large, prospective, multicenter cohort of infants.
Methods:
We studied 94 cholestatic infants enrolled up to 6 months of age in the NIDDK ChiLDReN (Childhood Liver Disease Research Network) “PROBE” protocol with a final diagnosis of IC; they were followed up to 30 months of age.
Results:
Male sex (66/94; 70%), preterm birth (22/90 with data; 24% born at < 37 weeks’ gestational age), and low birth weight (25/89; 28% born at <2500 g) were frequent, with no significant differences between outcomes. Clinical outcomes included death (n = 1), liver transplant (n = 1), biochemical resolution (total bilirubin [TB] ⩽1 mg/dL and ALT < 35 U/L; n = 51), partial resolution (TB > 1 mg/dL and/or ALT > 35 U/L; n = 7), and exited healthy (resolved disease per study site report but without documented biochemical resolution; n = 34). Biochemical resolution occurred at median of 9 months of age. GGT was <100 U/L at baseline in 34 of 83 participants (41%).
Conclusions:
Frequency of IC and of death or liver transplant was less common in this cohort than in previously published cohorts, likely because of recent discovery and diagnosis of genetic etiologies of severe/persistent cholestasis that previously were labeled as idiopathic. Preterm birth and other factors associated with increased vulnerability in neonates are relatively frequent and may contribute to IC. Overall outcome in IC is excellent. Low/normal GGT was common, possibly indicating a role for variants in genes associated with low-GGT cholestasis—this warrants further study.
What Is Known
- Cholestasis in neonates and young infants is commonly idiopathic.
- Prior studies, primarily small, retrospective, and performed in a single center, suggest that idiopathic cholestasis is often self-limiting.
What Is New
- We report data from a large cohort of infants with idiopathic cholestasis enrolled in a prospective, multicenter study.
- Male gender, preterm birth, and low birth weight are common compared with the general United States population.
- Outcomes have improved, and idiopathic cholestasis is diagnosed less frequently in this cohort compared with previously published cohorts.
- Low gamma-glutamyl transferase, present in many cases, suggests a role for variants in genes associated with low- gamma-glutamyl transferase cholestasis in some cases of idiopathic cholestasis.
Idiopathic cholestasis (IC), including idiopathic neonatal hepatitis (INH), is one of the most common diagnoses for infants with cholestatic jaundice (1.-4.). The term “INH,” specifically, is applied when etiology is unknown and histopathological features on liver biopsy are characteristic (giant cell hepatitis) and do not indicate another specific diagnosis (1., 5., 6.). Spontaneously resolving forms of IC have been attributed to a variety of environmental factors and extrahepatic insults, such as infections, cardiac dysfunction, and hypoxic/ischemic injury, affecting the highly vulnerable neonatal liver with its immature bile secretory mechanisms (7.-12.). Historically (in the early 1970s), most infants presenting with cholestasis were assigned a diagnosis of idiopathic neonatal hepatitis (Balistreri 2006). Advances in molecular understanding of cholestatic liver disease and enhanced detection of infectious and metabolic etiologies have, however, led to identification of the etiology in many infants and a significant reduction in the diagnosis of IC (3., 6., 13.-18.).
Studies published since the 1970s have suggested that the prognosis for IC (including INH) is generally good; however, most studies were retrospective, performed at a single center, and included no more than 70 infants with IC (3., 13.-19.). A large retrospective study reported disease course in 92 infants with IC but focused exclusively on those with transient disease (9.).
The Childhood Liver Disease Research Network (ChiLDReN), a National Institutes of Health-funded multicenter research consortium, has conducted a prospective longitudinal study of infants presenting with neonatal cholestasis at 15 clinical sites in the United States and Canada over an 11-year period. Here, we aim to describe demographic, historical, clinical, and laboratory indices at baseline and follow-up in a large cohort of infants with IC.
METHODS
Study Population
Participants with neonatal cholestasis enrolled in the prospective ChiLDReN “PROBE” protocol (Prospective Database of Infants with Cholestasis; NCT 61828) with a last known diagnosis of idiopathic/indeterminate cholestasis or INH were studied. The inclusion criteria (age <6 months, serum direct or conjugated bilirubin level ≥2 mg/dl and >20% of total serum bilirubin [TB]), and exclusion criteria for PROBE (including bacterial or fungal sepsis, ischemic hepatopathy, hemolytic disease, and total parenteral nutrition [TPN] cholestasis among other criteria) have been previously described (20.). Clinical evaluation and treatment of infants was conducted per the discretion of the treating physician at the clinical site, and the final diagnosis as determined at the study site was submitted to the database. Infants with biliary atresia or other defined diagnoses (as previously described) were excluded from this analysis (20.). The enrollment period was from June 1, 2004 through December 31, 2014 allowing all participants to have completed their scheduled follow-up to 24 months of age. A year 2 study visit was deemed completed for the purposes of our analysis if both TB and alanine aminotransferase (ALT) values were available for participants having a study visit between 21 and 30 months of age (which is the 2-year visit “window” as per the PROBE protocol). GGT values were included in the analyses as although they were inconsistently reported and upper limit of normal is substantially higher in young infants than in older infants, a low GGT value (ie, <100) in the setting of cholestasis may reflect a role for genes associated with so-called “low GGT cholestasis,” such as PFIC, in in the pathogenesis of cholestasis in this subset of participants. Infants discovered to have the following abnormal diagnostic results at the clinical site (all of the following are items included on the protocol Clinical Research Form) were excluded from this analysis: alpha-1 antitrypsin phenotyping (ZZ or SZ Pi type), sweat chloride test (≥30 mEq/L), cystic fibrosis neonatal screening or genetic testing, urine bile acid analysis, urine succinylacetone, thyroid function, galactosemia screening, JAG1 mutation testing. Clinical sites were not specifically queried for additional diagnostic test results.
Clinical Outcomes and Definitions
Biochemical resolution of IC was defined as TB ⩽1.0 mg/dL (17.1 μmol/L) and ALT <35 U/L tested at the same time point in participants without liver transplantation (21.-23.). Participants who met these criteria at any time point before or at the 2-year visit were classified in the biochemical resolution group, while participants with ALT ≥35 U/L based on the 2-year labs were classified in the partial resolution group. The term “partial resolution” was used as all of these participants normalized their TB (⩽1.0 mg/dL) but not ALT (all were ≥35 U/L) on 2-year labs. Gamma-glutamyl transferase (GGT) was not used as a criterion for biochemical resolution of disease because of substantial variability of normal range depending on laboratory and age as GGT elevation does not occur in some forms of cholestasis, and as GGT values were not consistently reported.
The primary clinical outcomes for this study were death, liver transplant, or achievement of biochemical resolution of disease (ie, biochemical resolution vs partial resolution) by the year 2 visit.
In this cohort, there was a group of participants who did not meet our defined criteria for biochemical resolution but were judged by the study site investigator to have resolved their liver disease (PROBE protocol definition states, “complete clinical and biochemical resolution of disease off all therapy”), and hence exited healthy from the study. These participants were classified in the exited healthy group.
Participants lost to follow-up were excluded from our analysis as it cannot be determined whether they had persistent or resolved disease by the year 2 visit.
Statistical Analysis
Demographics and baseline and follow-up laboratory measures through the 2-year visit were described using median and interquartile range or frequency and percent. Fisher exact and Wilcoxon tests were used to compare categorical and continuous variables, respectively. Primary analysis compared participants with biochemical resolution to those with partial resolution. A P value of <0.05 was considered significant. Due to singular instances for death and transplant, these participants were not considered for descriptive analysis.
In a separate sensitivity analysis, the stringency of the definition of recovery in the exited healthy group (participants reported as recovered by study site investigator) was reduced by combining the biochemical resolution and exited healthy groups, and we compared them with the partial resolution group.
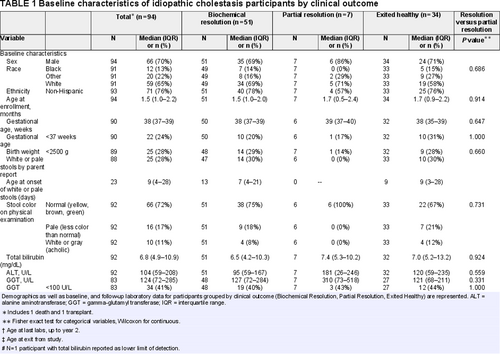
For exploratory purposes, we examined trajectories of primary laboratory markers over time. For each clinical outcome, we fit LOcally Estimated Scatterplot Smoothing (LOESS) curves along with 95% confidence intervals to depict patterns in lab values over age at lab test. The optimal smoothing parameter was chosen by the corrected Akaike Information Criteria (AIC) (24.). In Figure 1, 1 extreme outlier (with ALT ≈2500 U/L) was excluded from the LOESS curves, which resulted in a better model fit to the data.
LOESS curve for alanine aminotransferase by resolution status. Serum ALT of participants during the study period is shown. y-axis represents serum ALT (U/L) and x-axis shows the age of the patients (in months) at the time of the lab draw. Shaded areas represent 95% confidence intervals. Patients with biochemical resolution (circle) are compared with patients who did not have resolution (cross). ALT = alanine aminotransferase, LOESS = LOcally Estimated Scatterplot Smoothing.
RESULTS
Participant Outcomes
In total, 215 of 938 (23%) of infants enrolled in PROBE between June 1, 2004 and December 31, 2014 were characterized as having IC. Sixty participants were excluded from the analysis because of positive findings in diagnostic screening evaluations (details in “methods” section), and 61 participants were excluded because of no 2-year follow-up data. Ninety-four participants were included in this analysis (Fig. 1, Supplemental Digital Content, http://links.lww.com/MPG/C438); 1 participant died at 4.7 months of age, 1 underwent liver transplant at 9 months of age, 51 (54%) had documented biochemical resolution of disease on or before the 2-year visit, 34 (36%) were exited healthy without biochemical documentation of disease resolution, and 7 participants (7.4%) normalized TB but ALT remained ≥35 U/L (partial resolution group). Using a less restrictive definition of recovery in our secondary analysis (which combined the biochemical resolution and exited healthy groups), 85 participants (90%) recovered from IC.
The disposition and laboratory data for the 7 partial resolution group participants beyond our 30-month study window is described in Table 1, Supplemental Digital Content, http://links.lww.com/MPG/C442.
The participant who died at 4.7 months of age had multiorgan failure and “likely mitochondrial disease” per the autopsy report but further evaluation for mitochondrial disease was not pursued and this diagnosis was never confirmed. The participant who received liver transplant, at 9 months of age, had stage 3 to 4 fibrosis and features consistent with extrahepatic obstruction on liver biopsy at 3.9 months of age. MRI at 4 months of age showed hepatosplenomegaly, small extrahepatic bile ducts, nonvisualized intrahepatic bile ducts, and a cystic lesion near the porta hepatitis. Further clarification regarding diagnosis of this participant's disease was pursued from the clinical site where this participant received care, and final diagnosis was cited as inconclusive.
Demographics
The 94 participants were predominantly male (N = 66) overall as well as in each outcome subgroup (Table 1). A majority were white (N = 59 white; N = 12 black, N = 20 other), non-Hispanic (N = 71). Twenty-two of 90 (24%) with data available were born at <37 weeks’ gestational age. The earliest gestational ages were 29 weeks (the participant who died), and 30 weeks (exited healthy group). Low birth weight (<2500 g) was present in 25 of the 89 (28%) participants with birth weight reported.
Data at Enrollment
Nearly one-third of infants in the biochemical resolution (14/47 with data available) and exited healthy (10/33 with data available) groups had white or pale stools; none in the partial resolution group reported history of pale stools.
Serum TB levels (median) at enrollment were approximately 7 mg/dL for biochemical resolution, partial resolution, and exited healthy groups. The median ALT levels trended lower in the biochemical resolution group (95 U/L) than in the partial resolution group (181 U/L) (NS), as did the median values of baseline GGT (127 vs 310 U/L; NS). The proportion with so-called “low GGT cholestasis” (GGT <100 U/L), often observed in genetic disorders of intrahepatic cholestasis, was 41% (34/83) in participants with GGT tested (all outcome subgroups combined).
Family history was potentially relevant in a few participants (Table 2, Supplemental Digital Content, http://links.lww.com/MPG/C443). DNA is obtained from participants as part of the PROBE protocol, but genetic analysis is planned for a separate analysis and these data will not be discussed here.
Follow-up Data
At the last follow-up, TB levels were ⩽1.0 mg/dL in all participants in the biochemical resolution and partial resolution groups, with TB first reaching a value ⩽1 mg/dL at median 4.6 months of age in the biochemical resolution group and 5.2 months of age in the partial resolution group. ALT and GGT levels in the partial resolution group remained elevated at last follow-up; however, and were significantly higher than those in the biochemical resolution group: median 54 (vs 27 for biochemical resolution; P < 0.001) for ALT, and median 58 (vs 16; P = 0.004) for GGT. These laboratory values were measured at median ages of 9 and 24 months in the biochemical resolution and partial resolution groups, respectively. In the biochemical resolution group, ALT reached <35 U/L by a median 7.6 months of age. GGT was ⩽100 U/L in all except 2 participants with a recorded value at last follow-up in the biochemical resolution group. Median platelet counts, although somewhat lower in the partial resolution group (median 237 × 103/μl, interquartile range [IQR] 179–311) than in the biochemical resolution (median 337, IQR 257–446) and exited healthy (median 403, IQR 326–566) groups, medians were all well within normal range. At last available labs, there were only 2 participants with platelet count <150 × 103/μl, 1 from the biochemical resolution group, and the other from the partial resolution group.
All available TB, ALT, and GGT values, along with smoothing curves and confidence limits, provide further insight into laboratory trajectories for the biochemical resolution and partial resolution groups (Figs. 1-3). In examining the most rigorously defined subgroups, biochemical resolution and partial resolution, the most overlap between groups occurs for TB and ALT before 3 months of age, but, thereafter, the curves for partial resolution become higher (as might be expected) than the biochemical resolution curves. GGT levels are uniformly higher in the partial resolution than the biochemical resolution group between about 6 and 12 months of age. Although TB begins to drop steadily and immediately in both groups, there is an increase in ALT around 3 months of age in both groups, after which these begin to fall. There is a similar, slight increase in GGT around 3 months of age observed only in the biochemical resolution group. The curves appear similar when combining the exited healthy participants with the biochemical resolution group, as was done in the secondary (sensitivity) analysis (Figs. 2–4, Supplemental Digital Content, http://links.lww.com/MPG/C439, http://links.lww.com/MPG/C440, http://links.lww.com/MPG/C441).
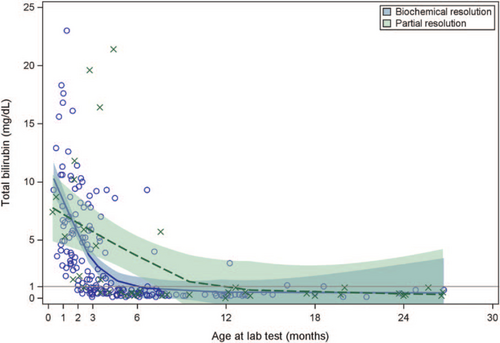
LOESS curve for total bilirubin by resolution status. The total bilirubin of participants during the enrollment period is shown. y-axis represents total bilirubin (mg/dL) and x-axis shows the age of the patients (in months) at the time of the lab draw. Shaded areas represent 95% confidence intervals. Patients with biochemical resolution (circle) are compared with patients who did not have resolution (cross).
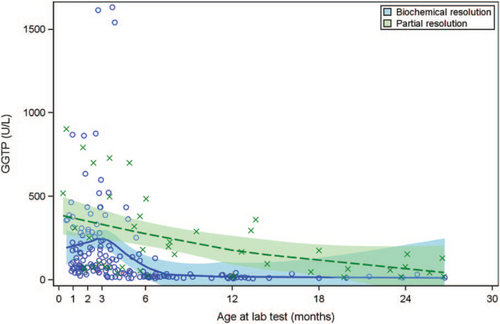
LOESS curve for gamma-glutamyl transferase by resolution status. Serum GGT of patients during the enrollment period is shown. y-axis represents serum GGT (U/L) and x-axis shows the age of the patients (in months) at the time of the lab draw. Shaded areas represent 95% confidence intervals. Patients with biochemical resolution (circle) are compared with patients who did not have biochemical resolution (cross). GGT = gamma-glutamyl transferase.
DISCUSSION
Herein, we describe demographic and clinical features and outcomes in a prospective, multicenter study of a cohort of infants with IC, and observe that the outcomes are excellent, with 98% alive with native liver by 30 months of age. Several older studies reported higher rates of death or transplant than we have observed—ranging from 7 to 31% (13., 14., 17.-19.). More recently, Hoerning et al (3.) cited no deaths in 11 cases of infantile IC included in a series of infants with a variety of cholestatic liver diseases; it is not noted if any of these infants underwent transplant. Likewise, older studies have demonstrated less frequent resolution of disease, ranging from 49 to 86%.
We have also observed a decrease in the frequency of IC as a diagnosis (of exclusion) in cholestatic infants (23% of all infants, 53% of non-BA infants) compared with previous reports. Earlier studies (1977–2001) have cited idiopathic etiology in 41% to 69% of all cholestatic infants including those with a diagnosis of BA (13.-18.). Subsequently, Hoerning et al (3.) in 2014 cited 13% (11 out of 82) of infants with intrahepatic or extrahepatic cholestasis as having idiopathic disease. This decrease, like the decrease we see in mortality compared with older studies, is also not unexpected, given advances in the diagnosis and in identification of infantile cholestatic liver diseases. The slightly higher frequency of IC in the present cohort compared with that reported by Maggiore et al could be explained, in part, by exclusion of infants from enrollment in PROBE (including malignancy, hemolytic disorder, birth weight less than 1500 g) that were included in that series; that is, our analysis may present a slight overestimation of the true frequency of IC among cholestatic infants receiving care at centers participating in the ChiLDReN (PROBE) study because of exclusion of these infants from our analysis (14.).
Pale stool color was not present in partial resolution infants., and thus, based on our cohort, does not appear to be associated with outcome, although it may be inappropriate to draw a conclusion based on this very small number of infants. Prior PROBE studies found that a disproportionately high number of cholestatic babies diagnosed with BA had acholic stools compared with nonobstructive cholestasis (including IC), indicating that increased vigilance is appropriate in the presence of acholic stools when biliary atresia has not yet been ruled out (20.). In contrast to other reports, however, if biliary atresia is not being considered and diagnosis remains idiopathic, our data suggest that acholic stools may not be associated with a poor outcome (5., 25.).
We observed a relatively high frequency of preterm births (< 37 weeks) in our cohort—24% overall, compared with 9.9% reported in the general population in the United States in 2016 (26.). Likewise, 28% of infants with birth weight data available were born at low birth weight (<2500 g), considerably higher than the 8.2% reported in the United States in 2016 (26.). This relatively high frequency of preterm and low birth weight in our cohort is similar to findings cited in previous studies of infants with IC, and supports a role for intrauterine factors and/or immature liver in the pathogenesis of IC (5., 13., 25., 27., 28.). It should be acknowledged that cholestatic infants presenting at our centers with birthweight <1500 g (unless diagnosed with BA), as stated above, were excluded from enrollment based on PROBE's exclusion criteria, which likely decreased, to some extent, the number of both pre-term and low-birth-weight cholestatic infants in our sample.
There are also a disproportionately high number of male infants in our cohort (70%), which is consistent with previous reports of infants with IC (13., 17.). The explanation for this observed sex difference is not known. A “male disadvantage” in survival of neonates has been described previously but an explanation for this phenomenon remains unknown after more than 40 years since it was first observed (29.). Newborn male infants are reported to have increased vulnerability of lung, brain, and circulatory function; there may be unexplained hepatic factors that are as-of-yet unidentified, or the liver may suffer from compromised function of other organ systems in male infants more than in female infants (30.).
We do not have detailed historical information specifically regarding perinatal hypoxememia/asphyxia or arrhythmia, which have been previously reported in association with transient cholestasis, although infants with hypoxia, shock, or suspected ischemic hepatopathy within the preceding 2 weeks were excluded from enrollment in PROBE, so our study likely underrepresents this previously cited susceptibility factor in neonatal cholestasis. The transient nature of biochemical abnormalities in most infants in our cohort; however, supports the hypothesis that fetal/neonatal events, in the setting of an immature liver, play an etiopathogenic role in development of cholestasis in these infants (8., 10., 31., 32.). Undiagnosed infection could also have played a role in the pathogenesis of transient cholestasis in our cohort (17.).
Another potential pathogenic factor that must be considered is the presence of heterozygous or “milder” homozygous variants in genes known to be causative for disorders, such as progressive familial intrahepatic cholestasis (“PFIC”) (14., 33.-38.). Almost half of this cohort, in all outcome subgroups, had a GGT <100 U/L at baseline. This could reflect genetic variants associated with genes causing so-called “low GGT cholestasis” in these infants. Normal GGT has been associated with lower likelihood of resolution of infantile cholestasis in 2 previous reports [Maggiore et al (14.) and Lu et al (39.)], and further investigation of a potential role for variants in genes associated with hereditary cholestasis in infants with “idiopathic” and/or transient neonatal cholestasis is needed; this analysis of genetics in our cohort is in progress.
In sum, in a multicenter, prospective study of infants with IC, we have observed excellent clinical outcomes that were improved compared with previous studies. We observed a decreased frequency of idiopathic diagnosis overall in cholestatic infants, as well. These findings reflect improved ability to broadly, rapidly, and (sometimes) inexpensively screen for genetic disorders, as well as identification of previously undiscovered disorders. High frequency of low birth weight and transient nature of disease suggests a perinatal insult (such as perinatal hypoxemia), along with immature bile secretory mechanisms, as a likely contributor to cholestasis in these infants. The rather frequent occurrence of GGT <100 U/L at enrollment in our cohort suggests the possible role of heterozygous variants in single or multiple genes associated with autosomal recessive intrahepatic cholestatic disorders. The caveat to our findings is that, given the rarity of IC, our sample size was modest, and findings should be interpreted with caution. Further studies, ideally in large cohorts, will provide further insight.