Mycophenolate Mofetil Hepatotoxicity Associated With Mitochondrial Abnormality in Liver Transplant Recipients and Mice
The mouse study was supported in part by R21NS100077 and R01NS089815 (A.T.S.).
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's Web site (www.jpgn.org).
An infographic is available for this article at: http://links.lww.com/MPG/C361.
ABSTRACT
Objectives:
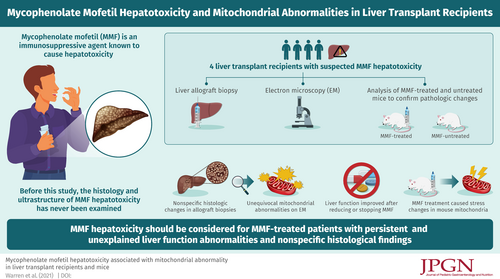
Mycophenolate mofetil (MMF) is a widely used immunosuppressive agent. MMF hepatotoxicity has been reported in non-transplant and renal transplant patients with minimal histologic description. This is the first study describing detailed histology and ultrastructure of MMF hepatotoxicity.
Methods:
Four liver-transplant recipients (Cases 1–4) were suspected to have MMF hepatotoxicity. Cases 1–3 (two females and one male; 4–17 years) had multiple biopsies for liver function test (LFT) abnormalities. Case 4 (female; 16 years) had a surveillance biopsy. Electron-microscopic examination (EM) was requested on Cases 1–3 for unexplained, persistent LFT elevation and histologic abnormalities despite therapy and Case 4 for unexplained histologic abnormalities despite a stable clinical course. To confirm the pathologic changes in the human allografts, livers from MMF-treated and untreated mice were also reviewed.
Results:
While the allograft biopsies showed nonspecific histologic changes, EM revealed unequivocal mitochondrial abnormalities similar to those seen in primary and secondary mitochondrial disorders. In Cases 1 and 2, LFTs improved after stopping and reducing MMF, respectively. In Case 3, pre- and post-MMF treatment biopsies were performed and only the post-MMF biopsy demonstrated mitochondrial abnormalities. Mitochondrial abnormality in Case 4 was subclinical. The mouse study confirmed that MMF caused various stress changes in the mitochondria; number of mitochondria/cell (mean ± standard deviation; untreated group: 58.25 ± 8.426; MMF-treated group: 76.37 ± 18.66), number of lipid droplets/cell (untreated: 0.9691 ± 1.150; MMF-treated: 3.649 ± 4.143) and sizes of mitochondria (μm, untreated: 0.8550 ± 0.3409; MMF-treated: 0.9598 ± 0.5312) were significantly increased in hepatocytes in the MMF-treated mice compared with the untreated mice (P < 0.0001).
Conclusions:
Although MMF is safe for the majority of patients, MMF can cause mitochondrial stress, which may trigger more severe mitochondrial abnormalities in a small subset. MMF hepatotoxicity should be considered for MMF-treated patients with unexplained, persistent LFT abnormalities and nonspecific histologic findings. EM should be requested for these cases.