Endoscopic Pancreatic Function Testing (ePFT) in Children
A Position Paper From the NASPGHAN Pancreas Committee
Nishant Patel, Zachary M. Sellers, Maisam Abu-El-Haija and Sohail Z. Husain are equal contributors.
The authors report no conflicts of interest.
This article has been developed as a Journal CME and MOC Part II Activity by NASPGHAN. Visit https://learnonline.naspghan.org/ to view instructions, documentation, and the complete necessary steps to receive CME and MOC credits for reading this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's Web site (www.jpgn.org).
ABSTRACT
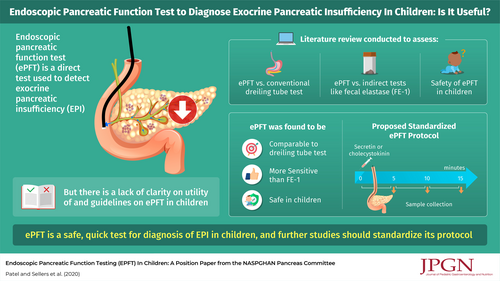
Endoscopic pancreatic function testing (ePFT) is one of the few ways to directly diagnose exocrine pancreatic insufficiency, and considerable confusion regarding indications, utility, and interpretation of the test remains. This position paper of the Pancreas Committee of the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition reviews the history and indications for ePFT in children. We compare various methods in current practice and determine their strengths and limitations, and based on data from children and adults we provide guidance on a protocol on how to perform ePFT in children. Lastly, we pose areas in need of further research relating to ePFT in children.
Graphical Abstract
An infographic is available for this article at: http://links.lww.com/MPG/B961.
Endoscopic pancreatic function testing (ePFT) is not universally performed across pediatric gastroenterology practices, yet there are a number of pediatric gastroenterologists who perform this procedure for a variety of indications. Interest regarding ePFT is increasing. The purpose of the present document is to review pancreatic exocrine function, various methodologies used to assess exocrine pancreatic function, the past and current status of ePFT, concepts, indications, variations, and controversies with ePFT. Finally, based on our collective experience, we provide guidance on performing ePFT in children, including a sample protocol for clinicians to use. We then highlight areas of future research that will advance the standardization, application, and utilization of ePFT in pediatric care.
THE ONTOGENY OF PANCREATIC ENZYME SECRETION
The exocrine pancreas is essential for proper nutrient digestion. The main parenchymal cell of the pancreas, the acinar cell, is responsible for the synthesis of digestive enzymes. Ductal cells, which line an intricate outflow ductal system, secrete bicarbonate and water to form pancreatic fluid in its final form. Pancreatic secretions are essential for catalyzing dietary carbohydrates, proteins, and especially fats. Zymogen granules, which are densely packed vesicles at the apical, or luminal, pole of acinar cells, appear between 14 and 16 weeks of gestation (1., 2.). Several of the digestive enzymes have a distinct ontogeny. For example, pancreatic lipase-related proteins are present at 16 weeks gestational age, while triglyceride lipase is not detected in the fetal pancreas (3.). Infants are labelled as having “physiological” steatorrhea in the first 3–6 months of postnatal life, since overall lipase output is 5–10% of adult values (4.-6.). Similarly, amylase is not detectable until 39 weeks gestational age (1., 2.), and functionally active amounts of amylase do not arise until the postnatal age of 6 weeks (5., 7.-10.). The postnatal maturation of pancreatic enzymes reflects the changes in an infant's diet during the first year of life. The relative immaturity of the exocrine pancreas in infants can, however, render infants vulnerable to situations of metabolic or nutritional stress (11.). Knowledge of the ontogeny of pancreatic enzyme availability in pancreatic juice can also be useful when considering normal age-based values of pancreatic enzyme activity levels in duodenal fluid samples during pancreatic function testing.
HORMONAL CONTROL OF DIGESTIVE ENZYME SECRETION
Digestion occurs in four major coordinated phases: the cephalic phase, which is heralded by the act of perceiving, smelling, seeing, or tasting food; the gastric phase; pancreatic phase; and intestinal phase. Early during digestion, the salivary glands are stimulated to secrete α-amylase, which begins the digestion of starch into sugars, a process that is accelerated by pancreatic amylases. During the gastric phase, pepsins in the hydrochloric acid-rich gastric juice begin the breakdown of proteins into peptides, and gastric lipase processes the digestion of fats. The pancreatic phase is regulated by several neurohormones, particularly cholecystokinin (CCK) and secretin (12.). The major roles of CCK are to induce the secretion of pancreatic enzymes from the acinar cell zymogen granules and to stimulate gallbladder contraction with release of bile into the duodenal lumen. Secretin stimulates bicarbonate and fluid secretion from the ductal cells into the ductal lumen. Most of the pancreatic digestive enzymes are proenzymes, or zymogens, and are activated in a cascade, initially through the activation of trypsinogen to trypsin by enterokinase along the intestinal brush border, followed by activation of the other zymogens by trypsin. The optimal pH in the duodenum for zymogen activation occurs at a range of 7.8–8.7 (13.). Between meals, there is a constitutive secretion of pancreatic juice. In healthy adult volunteers, this resting fluid output reaches 20–25% of maximum (14., 15.). In addition, occasional bursts of pancreatic secretion are controlled by the migrating myoelectric complex (16.).
EXOCRINE PANCREATIC INSUFFICIENCY AND INDICATIONS FOR PANCREATIC FUNCTION TESTING
Exocrine pancreatic insufficiency (EPI) is defined as a decrease from normal values of pancreatic enzyme or bicarbonate secretion, or both (17.), in the small intestine that interferes with adequate digestion and absorption of ingested nutrients. Cystic fibrosis is the most common cause of EPI in children (18.). Other congenital causes include Shwachman-Diamond syndrome, Johanson-Blizzard syndrome, Pearson marrow pancreas syndrome, and Jeune syndrome. Other rare causes of EPI are due to aberrant embryonic development of the pancreas, and some of the known congenital causes likely arise from intense burnout of the inflamed pancreas in utero(19.). Acquired causes of EPI can be transient, such as in the aftermath of acute pancreatitis (which can persist weeks to months) (20., 21.), acute gastroenteritis, and malnutrition (22.), or they can be irreversible, such as from parenchymal destruction during chronic pancreatitis or surgical resection of the pancreas. In addition to villous blunting, celiac disease can cause steatorrhea as a result of EPI. The mechanism for this has not been well elucidated, but may be due to several possibilities, including, but not limited to, a decrease in the number of intestinal CCK and secretin-secreting, I and S cells, respectively, concomitant chronic pancreatitis, pancreatic atrophy, and/or impaired absorption of substrates necessary for synthesis of pancreatic enzymes (23.). Considerations of EPI etiology are important when considering the optimal timing of performing EPI testing.
The common indications for pancreatic function testing in children are listed in Table 1. The value of any test for pancreatic function is to establish a diagnosis of whether maldigestion is of pancreatic origin or not to help direct treatment and monitoring of complications of disease. Pancreatic enzyme replacement therapy (PERT) is the most common treatment for EPI; however, it may not be the necessary treatment (or only necessary treatment) for nonpancreatic causes of maldigestion or isolated deficiencies in pancreatic enzymes. Intestinal causes of malabsorption may require dietary changes (eg, celiac disease, sucrase-isomaltase deficiency) or immunomodulating drugs (eg, inflammatory bowel disease). Isolated pancreatic enzyme deficiencies may be improved with specific dietary interventions ± PERT (eg, limiting carbohydrates in isolated amylase deficiency). Empiric PERT without a diagnosis may sometimes be indicated, but should be used cautiously. Comprehensive reviews on pancreatic function testing in children have recently been published, including as a joint NASPGHAN/ESPGHAN report (17., 24.). Pancreatic function testing is divided into two broad groups: indirect (nonstimulatory) and direct (stimulatory) testing (Table 2).
INDIRECT PANCREATIC FUNCTION TESTING
Indirect tests are assayed from various sources, including stool (eg, fecal fat testing, fecal assays for the digestive enzymes elastase-1 or chymotrypsin), serum (eg, tests for immunoreactive trypsinogen, fat-soluble vitamins, vitamin B12, or essential fatty acids), breath (malabsorption breath test or 13C-mixed triglyceride breath test), and urine (pancreolauryl testing). A malabsorption blood test using serum lipid profiling is also under development for clinical use (25.). All of these tests examine baseline, constitutive, nonstimulated pancreatic secretion, and most of them, other than the fecal or serum digestive enzyme assays, indirectly assess the downstream effects of EPI. The benefit of the indirect pancreatic function tests is that they are noninvasive, but currently commercially available ones detect only severe EPI. Indirect testing is less sensitive and specific compared with direct function tests. The newer modalities, such as the breath tests, offer promise, but they are currently cumbersome and/or not widely available.
DIRECT PANCREATIC FUNCTION TESTING: THE ORIGINAL DREILING TUBE AND ENDOSCOPIC PANCREATIC FUNCTION TESTING
Direct pancreatic function tests aim to “directly” measure pancreatic function by exogenously stimulating pancreatic enzyme and fluid secretion using CCK or secretin, respectively. One of the first direct stimulatory pancreatic function tests, described in 1948, was the Dreiling tube test (26.). The test involves placing a duodenal tube under fluoroscopy past the ampulla of Vater. Duodenal fluid is continuously aspirated and captured in timed aliquots, usually over a 60- to 90-minute duration, after intravenous administration of pancreatic stimulating hormones. The key measurements of direct pancreatic function are volume aspirated, bicarbonate concentration, and the activities of various pancreatic enzymes. To prevent blunting of pancreatic enzyme activity from gastric acid, the catheter houses an extra lumen with a gastric port for constant aspiration of gastric secretions. To determine pancreatic fluid output, a nonabsorbable marker is constantly infused through a third lumen, whose end is situated at the proximal duodenum. The concentration of the marker is measured from each of the aliquots, and the degree of dilution is used to back-calculate a corrected amount of pancreatic fluid secretion. While the Dreiling tube test has been considered a gold standard for identifying EPI, it has major practical limitations. Tube placement is cumbersome and requires fluoroscopy. The testing itself is laborious, uncomfortable, and time consuming. As a result, only a handful of publications applied the Dreiling tube test in children (24.), and, to our knowledge, few, if any, centers routinely employ the test.
Given the complexities of performing the Dreiling tube test, a direct pancreatic function test through an endoscope, or ePFT, was developed. The ePFT is performed during a standard esophagogastroduodenoscopy (EGD) (17., 24.). The patient may be in any position; however, placement in the left lateral decubitus position may help decrease contamination with gastric fluid and allow pancreatic secretion to be more readily suctioned from the dependent portion of the duodenum. At the start of the procedure, the patient is infused with either secretin (0.2 μg/kg, max: 16 μg) or CCK (0.04 μg/kg bolus or 0.02–0.04 μg · kg−1 · hr−1), or both, depending on institutional protocols, preference, and/or availability. The endoscope is passed through the esophagus, and the stomach is emptied of gastric contents. Secretions from the second portion of the duodenum near the ampulla of Vater are collected in 3–4 sequential aliquots. The samples may be analyzed for pH, electrolytes, protein content, amylase, lipase, trypsin, chymotrypsin, and elastase, either locally on fresh specimens or subsequent to freezing and sending out for external analysis. The highest enzyme activity for each of the aliquots is reported as the final value (in activity units · mL−1 · min−1 or nmol · mL−1 · min−1).
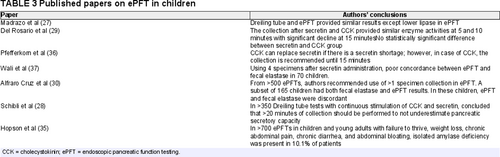
One of the earliest reports to compare ePFT with the Dreiling tube method was published in 1991, in which 35 toddlers with chronic diarrhea or failure to thrive underwent 1 of the 2 tests (27.). The Dreiling tube test was with CCK followed by secretin with duodenal aspiration every 10 minutes for 1 hour. The total procedure time of the Dreiling tube test was an average of 3 hours. The ePFT was administered only with secretin, and the test lasted an average of 45 minutes with duodenal aspiration every 10 minutes. The enzyme activity levels were comparable except for lower lipase levels with secretin compared to CCK stimulation. Notwithstanding the difference that the Dreiling arm administered both CCK and secretin, while the ePFT arm only utilized secretin, the authors concluded that endoscopic collection of duodenal fluid following secretin is a safe, less time-consuming, and reliable method for assessing pancreatic function in children.
CONTROVERSY IN THE PEDIATRIC LITERATURE BETWEEN THE DREILING TUBE TEST AND ENDOSCOPIC PANCREATIC FUNCTION TESTING
The accuracy and appropriateness of performing direct pancreatic function tests in children is not without controversy. In 2006, Schibli et al compared data using the full 60 minutes of sampling through a Dreiling tube test with data, also from the Dreiling tube, but without the use of perfusion markers and from only the first 20 minutes of collection (28.). The authors suggested that the latter mimicked conditions of the ePFT. Data were from pre-existing records of Dreiling tube tests performed in children during a span of 25 years at the Hospital for Sick Children in Toronto. The conclusions were that the full version of the Dreiling tube test was superior. The authors argued that the use of a marker is necessary to control for the variability in the recovery of secretions. However, the perfusion markers yielded a mean recovery rate of pancreatic secretions that tightly clustered around 55–60%. Thus, it was proposed that a constant correction factor could be incorporated into a calculation for enzyme output, without the need for a perfusion marker. Schibli et al also noted that shortening the duration of sampling to 20 minutes from 60 minutes missed the maximal rate of enzyme output in many of the cases. However, the duration of sampling is not a fundamental difference between the two tests, and several studies of ePFT in both children and adults, described in the next section, demonstrate that the peak enzyme activities and bicarbonate level were achieved within 20 minutes of starting the collections and as early as 5 minutes in the enzyme activity calculations, when there was more rapid stimulation with secretin alone (24., 29.-33.). The study above highlights that the field is still in need of studies to help develop the optimal method for ePFT in children.
THE LITERATURE ON ENDOSCOPIC PANCREATIC FUNCTION TESTING IN CHILDREN
Multiple pediatric studies have investigated the utility and protocols of ePFT in evaluation of children for EPI (Table 3). The use of ePFT in children gives results comparable to the Dreiling tube method.
In 2000, Del Rosario et al (29.) compared the enzyme values of pancreatic secretions in children via ePFT collected every 5 minutes for 15 minutes after receiving CCK followed by secretin versus secretin alone. They noted the enzyme level peaks at 5 minutes and continues beyond 10 minutes; however, the enzyme activity per mL of fluid declines after 10 minutes due to water and ion (bicarbonate) secretion. Based on these findings, the authors concluded that pancreatic fluid collected within 5–10 minutes after secretin administration allows the diagnosis of EPI regardless of whether it is generalized insufficiency or an isolated enzyme deficiency (34., 35.). In 2002, due to a shortage of secretin in the United States, Pfefferkorn et al (36.) evaluated the use of CCK alone for ePFT versus the combination of CCK and secretin or secretin alone. They concluded that CCK can be used as a single agent for direct pancreatic enzyme measurements. The fact that secretagogues produce comparable enzyme activity levels with the use of CCK alone, secretin alone, or in combination allows the providers to choose from what they have available in their endoscopy units to produce similar results. It is not known if the same applies to using different secretagogues when measuring bicarbonate levels, since secretin is responsible for bicarbonate and water secretion. Furthermore, ePFT studies in children have been conducted to evaluate the performance of routinely obtained indirect pancreatic function testing, fecal elastase-1 (FE-1), with the results of direct testing by ePFT (37.). In 2012, Wali et al examined the correlation between FE-1 and ePFT-obtained elastase in 70 children and showed poor concordance between elastase values measured by FE-1 and ePFT (r = 0.190). All 11 children with abnormal ePFT elastase had FE-1 ≥200 μg elastase/g stool (37.). Alfaro Cruz et al also reported a significant discordance between ePFT and FE-1 in a larger comparison study including 165 children, further highlighting the poor correlation between direct and indirect testing (30., 31.). That same study advocated for multiple sample collections to avoid false negative and false positive results and included data on enzyme maturation with age to set the standards for enzyme activities in the growing child (30.).
ASSAY METHODS AND DIFFERENCES IN DUODENAL COLLECTION AND STORAGE OF ePFT
The pH, bicarbonate concentration, protein content, amylase, lipase, trypsin, chymotrypsin, and elastase and other enzymes (eg, carboxypeptidase A and B) can be assayed from the collected fluid samples. Any sample with a pH less than 7 may be unreliable as it is below the pH optimum of the enzymes and may reflect contamination with gastric fluid (13.). Alternatively, the inability to increase pH, or bicarbonate, upon secretin stimulation may be reflective of loss of cystic fibrosis transmembrane conductance regulator (CFTR) function, through gene mutations in CFTR or other signaling pathways that involve CFTR, ductal damage, or smoking, which drives pancreatic ductal bicarbonate secretion. Different enzyme assay methods have been used in the published pediatric papers (27., 29., 36., 37.). Age-specific normative values for children have not been validated. Individual laboratory normative values should always be consulted, but those from a commonly used laboratory (Kaleida Health Children's Hospital Laboratory, Buffalo, NY) are: trypsin >55.4 nmol · mL−1 · minute−1, amylase >32 μmol · mL−1 · minute−1, lipase >146 μmol · mL−1 · minute−1, and chymotrypsin >2.5 μmol · mL−1 · minute−1. If performing ePFT in very young children, the effect of age-related maturation should be considered when evaluating enzyme levels (30.). Because most pancreatic proteases are sensitive to degradation, the fluid samples should be promptly placed on ice or dry ice. Fluid should be analyzed within 6 hours of collection if not frozen (38.). There is an excellent correlation of enzyme activity between fresh and frozen samples (r = 0.96) (5., 39.).
The conventional method to measure bicarbonate was back-titration of the pancreatic fluid, with defined quantities of hydrochloric acid until prespecified pH is obtained. It is a time-consuming process. Currently, most clinical laboratories have an autoanalyzer for bicarbonate measurement. There is a good correlation between the two methods (40.) (Lin's concordance coefficient = 0.96). Fluid for bicarbonate analysis does not need to be collected on ice (nor is it adversely affected if it is); however the container should be tightly sealed to prevent CO2 equilibration with ambient air. In healthy adult volunteers, the mean peak bicarbonate concentration in secretin-stimulated pancreatic fluid from nonpancreatitis controls was 103 ± 11 mEq/L. A cutoff point of 80 mEq/L was 2 standard deviations below the mean and considered abnormal (41., 42.). To date no studies have been published evaluating secretin-stimulated bicarbonate concentrations in children with or without EPI. Thus, whether bicarbonate concentration during pediatric ePFTs is useful or not and what the normal cutoff should be for children of different ages remain to be determined.
SAFETY OF ENDOSCOPIC PANCREATIC FUNCTION TESTING
ePFT is technically a straightforward procedure to perform in children. The procedure requires sedation for endoscopy and therefore carries the standard risk for both anesthesia and EGD. It is important to emphasize that sedation utilized for upper endoscopy appears to have no significant influence on the results of the procedure (43.). Secretin can cause a mild increase in heart rate with an average rise of 7 beats/min and also a mild elevation of systolic and diastolic blood pressures, but none of these changes are clinically significant (9.). The serum half-life of intravenously administered secretin is 4 minutes (44.). There are currently no contraindications to the use of human secretin, and pork allergy applies only to the porcine-derived secretin (9.). Secretin can be used safely during acute pancreatitis, if needed. Adverse events have not been reported with CCK in pediatric studies (27., 29., 36.).
Several medications influence pancreatic secretions in humans. Morphine increases bicarbonate and decreases enzyme secretion (45.). Glucagon suppresses pancreatic enzyme output and, to a lesser extent, bicarbonate (46.). Diazepam with hyoscine butylbromide reduces secretion of trypsin and delays appearance of bilirubin in the duodenal aspirate (47.). Low-dose atropine (5 μg/kg) decreases both basal and secretin-stimulated bicarbonate secretion (48.). The impact of these medications on the sensitivity and specificity of ePFT for diagnosing EPI in children needs to be studied further.
STANDARDIZED ENDOSCOPIC PANCREATIC FUNCTION TESTING PROTOCOL
Several centers employ ePFT, yet protocols vary, which has limited the acceptability of this test and the ability to make firm conclusions when comparing results obtained at different sites. Controversies remain with single or combination use of CCK and secretin. Meaningful comparisons of multicenter data will require standardization of the administration and sequence of hormone administration and the timing, frequency, and duration of duodenal fluid collection. Although further research is required to establish a universally accepted protocol for ePFTs in children and recognizing that the development of a common protocol is a key requirement to improve the utility and reliability of ePFTs in children, we have created a sample ePFT protocol for use in children (Fig. 1). The protocol is a set of instructions and suggested methodology based on the above literature review and our collective experience and may need further refining as pediatric-specific data are obtained. Variations may be considered based on the primary purpose of the test.

Sample ePFT protocol for determination of EPI in children. A. Flow diagram shows the steps involved in performing ePFT. Variations in patient position, use of suction catheters, secretin or CCK secretagogue, and measurement of pH, pancreatic enzymes, and/or bicarbonate concentration may vary by provider/institution. B. Sample protocol schematic. *Potential differences in the kinetics of enzyme secretion, pH, and bicarbonate secretion between secretin and CCK administration may alter time course of study. **Additional time periods may be necessary if measuring bicarbonate secretion. C. Setup and procedural details for sample protocol. 1If measuring bicarbonate concentration, remove an aliquot of fluid to be placed in a well-sealed second tube in order to send to chemistry lab. Note: you may need to make a notation to the laboratory that dilution may be necessary to ensure proper readout within range of the analyzer. CCK = cholecystokinin; EPI = exocrine pancreatic insufficiency; ePFT = endoscopic pancreatic function testing.
CONCLUSIONS AND FUTURE DIRECTIONS
The ePFT can be a valuable test for EPI to complement the use of nonstimulatory pancreatic function tests. It is a safe procedure that can be performed when routine EGD is performed for investigation of children suspected of having pancreatic exocrine dysfunction. Acinar function can be assessed for isolated enzyme deficiency or generalized deficiency when other noninvasive tests are inconclusive. Although the Dreiling tube has been considered “the gold standard” for direct pancreatic function testing in the past, ePFT is now preferred due to relative technical ease, shorter duration, and comparable efficacy. Pediatric-specific considerations include the potential need for age-specific interpretation, especially for infants and toddlers, as a result of enzyme maturation occurring throughout the early years of life. Additionally, the utility of bicarbonate concentration as a marker of chronic pancreatitis and/or EPI in children remains unknown. A multicenter study is needed for the standardization of ePFT in children with prospective collection of a large number of patients of all ages undergoing ePFT utilizing a single protocol. We have provided a potential protocol to move towards a universally accepted ePFT protocol in children. Optimizing this protocol, while minimizing total procedure duration, will maximize the benefits of ePFTs while minimizing risk for pediatric patients. The guiding principles we have outlined should facilitate safe and effective utilization of ePFT in children until sufficient pediatric-specific data exist that will lead to evidence-based best practices for ePFT use in children.