Reduced Intestinal Inflammation With Lumacaftor/Ivacaftor in Adolescents With Cystic Fibrosis
Source of funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Raphaël Enaud received a PhD grant from the University Hospital of Bordeaux for his research work.
Conflicts of interest: S.B., F.B., M.F., R.E. conduct clinical trials with Vertex pharmacological agents, on behalf of the European Cystic Fibrosis Society—Clinical Trials Network (ECFS-CTN) and within the scope of ECFS-CTN activities. L.D. received research grant from Vertex Pharmaceuticals to perform Lum-Iva-Biota study (this study is unrelated to the current manuscript). Vertex Pharmaceuticals had no involvement in the design of the present study and collection, analysis, and interpretation of data as well as in writing the manuscript. The remaining authors have no conflicts of interest relevant to this article to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's Web site (www.jpgn.org).
ABSTRACT
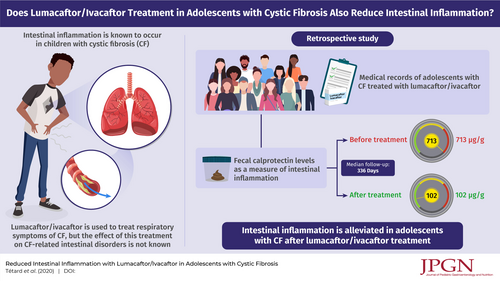
A chronic intestinal inflammation may occur in patients with cystic fibrosis (CF), while no therapeutic management is proposed. Although Lumacaftor/Ivacaftor is well-known to modulate the defective cystic fibrosis transmembrane conductance regulator (CFTR) protein in lungs, no data are available on the impact of this treatment on CF intestinal disorders. We, therefore, investigated the evolution of intestinal inflammation after initiation of Lumacaftor/Ivacaftor in CF adolescents (median of follow-up: 336 days [IQR: 278;435]). Median fecal calprotectin concentrations decreased significantly after Lumacaftor/Ivacaftor initiation (102 μg/g [IQR: 69–210]) compared with the baseline (713 μg/g (IQR:148–852), P = 0.001). To our knowledge, this study showed for the first time that CF-related intestinal inflammation is improved by Lumacaftor/Ivacaftor treatment.