Management of Juvenile Polyposis Syndrome in Children and Adolescents
A Position Paper From the ESPGHAN Polyposis Working Group
S.C. and W.H. received support in training in EBM learning by ESPGHAN 2016. C.D. was supported by the Zane Cohen Centre for Digestive Diseases.
The authors and the ESPGHAN Polyposis Working Group report no conflicts of interest.
ABSTRACT
The European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Polyposis Working Group developed recommendations to assist clinicians and health care providers with appropriate management of patients with juvenile polyposis. This is the first juvenile polyposis Position Paper published by ESPGHAN with invited experts. Many of the published studies were descriptive and/or retrospective in nature, consequently after incorporating a modified version of the GRADE system many of the recommendations are based on expert opinion. This ESPGHAN Position Paper provides a guide for diagnosis, assessment, and management of juvenile polyposis syndrome in children and adolescents, and will be helpful in the appropriate management and timing of procedures in children and adolescents. The formation of international collaboration and consortia is proposed to monitor patients prospectively to advance our understanding of juvenile polyposis conditions.
What Is Known
- There are no prior published guidelines specifically for children at risk, or affected by juvenile polyposis syndrome.
- In paediatric practice, timing of diagnosis, age, and frequency of endoscopy are not standardized, and will vary across clinicians, and between different countries.
- Currently clinical practice is based on case series and the clinicians’ personal exposure to juvenile polyposis patients.
What Is New
- European Society for Paediatric Gastroenterology, Hepatology and Nutrition polyposis working group made recommendations based on the literature and expert opinion.
- The recommendations are specifically on diagnosis, assessment, screening, and treatment of juvenile polyps in children and adolescents.
- This represents a practical guide for the management of patients with juvenile polyposis syndrome.
Juvenile polyposis syndrome (JPS) is a rare autosomal dominant precancerous condition associated with an increased risk of gastrointestinal (GI) tract cancers. Patients frequently present in childhood with rectal bleeding, but presentation may be related to extraintestinal manifestations, which can have significant health implications. Clinical classification of JPS is based on the number (≥5) and distribution of polyps. JPS can be diagnosed genetically, if a pathogenic germline variant is found; such mutations are found in up to 60% of patients with JPS. Rarely an infant can present with a severe juvenile polyposis phenotype identified due to diarrhoea, failure to thrive, and hypoalbuminemia.
The aim of this evidence-based and consensus-based position statement, commissioned by the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) is to provide a comprehensive review of the diagnosis and management of JPS in children. The goal is to make recommendations regarding age to screen at risk children, determine what surveillance is recommended based on apparent risk of cancer, role and timing of colonoscopy, and genotype-phenotype correlations. This position statement will address the issue of JPS diagnosis, based on published literature. Literature from PubMed, Medline, Embase, and Cochrane was reviewed and in the absence of evidence, recommendations reflect the opinion of paediatric and adult experts involved in the care of patients with JPS. Many of the published studies were descriptive and/or retrospective in nature, consequently after incorporating a modified version of the Grading of Recommendations Assessment, Development and Evaluation recommendation system many of the recommendations are based on expert opinion.
There are international efforts, collaboration, and consortia in other paediatric polyposis syndromes including constitutional mismatch repair deficiency syndrome (previously referred to as biallelic mismatch repair deficiency syndrome) (1.-3.). This undertaking is the first Position Paper published on JPS in the paediatric population. This Position Paper represents a starting point from which diagnostic and management decisions can undergo rigorous evaluation.
METHODS
ESPGHAN commissioned position papers on polyposis syndromes in 2016. Three task force leaders (S.C. and C.D. for JPS, W.H. for familial adenomatous polyposis [FAP], and A.L. for Peutz–Jeghers syndrome) invited the listed authors to participate in the project. The key questions were prepared by the coordinating team working group in face to face meetings in 2016 and 2017 and then approved by the other members. Each task force performed a systematic literature search to prepare evidence-based and well-balanced statements on their assigned key questions. Searches were performed in PubMed and/or EMBASE and/or Medline and/or Cochrane (publication year from 1997 to 2017) or before if needed, including as a minimum the key words “paediatric,” or “adolescent” or “children” and “juvenile polyposis syndrome” and “juvenile polyp (JP).” When insufficient information or publications were available in specific paediatric or adolescent papers then the search was broadened to include publications regarding adult patients. References in these documents were also searched to ensure acquisition of relevant source data. Clinical guidelines, systematic reviews, clinical trials, cohort studies, case-control studies, diagnostic studies, case series, and reviews after 1997 were all read.
A total of 589 abstracts were collected. After exclusion of 359 abstracts mainly for clear irrelevance to the topic, 230 full text manuscripts were retrieved and circulated to the relevant group participants. After excluding those articles defined as review, case reports, or others, finally 78 manuscripts were left for review.
JPS is a rare condition and therefore there was a paucity of good quality paediatric series. In the absence of evidence we relied on consensus of expert opinion and personal practice of the authors. To further reduce bias from individual practice, additional expert opinion on recommendations for managing this condition, was sought from physicians working within large established, polyposis registries with large cohorts of patients with JPS.
All articles studying JPS and JP in the age range were selected by title or abstract. The abstracts and then the full publications were reviewed. Most articles were not amenable for consistent grading by the level of evidence and strength of recommendation according to the Grading of Recommendations Assessment, Development and Evaluation system. Many articles were case series, with its attendant report bias. International guidelines were sought, and their evidence and referenced articles were also assessed. Each task force proposed statements on their assigned key questions which were discussed by email exchange or face-to-face meetings and voted on during the subsequent year. In April 2018, a draft prepared by S.C. and C.D. was sent to all group members and then subsequently modified. In ESPGHAN 2018, all members of the faculty discussed and reworded the final manuscript and voted on the statements included in this article.
WHAT IS JUVENILE POLYPOSIS SYNDROME?
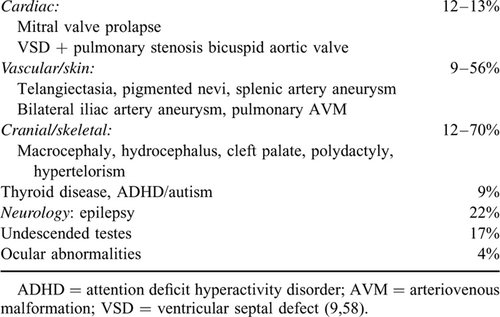
JPS (OMIM 174900) is a rare condition affecting between 1 in 100,000 and 1 in 160,000 people (4., 5.), and is inherited in an autosomal dominant manner. It is characterized by the development of multiple hamartomatous polyps of the GI tract. Although histologically distinct from Peutz-Jeghers polyps, hamartomas similar to that in JPS can be seen in the PTEN hamartoma tumour syndrome (PHTS) and are a characteristic of hereditary mixed polyposis syndrome. The GI tract phenotype is variable and extraintestinal manifestations can occur in patients with JPS (Table 1).
JPS is diagnosed by use of the following criteria in the absence of extraintestinal features consistent with PHTS (Cowden syndrome [CS] or Bannayan-Riley-Ruvalcaba syndrome) (6.):
- Five or more JPs of the colon or rectum, or
- JPs in other parts of the GI tract, or
- Any number of JPs and a positive family history.
JPs are hamartomatous polyps with normal epithelium, an inflammatory infiltrate with dilated, mucus-filled cystic glands in the lamina propria. Clinicians should be aware that pathology nomenclature can vary between pathologists and it can be helpful to request a pathology review of the polyps or pathology consultation by a pathologist experienced in reviewing challenging polyp cases.
Histologic features of adenomas are typically not seen in JPs. The term “juvenile” refers to the histopathology of the polyp and not the age of onset of polyps.
JPS may also be subdivided into 2 phenotypic groups according to clinical presentation and disease course (7.):
- Juvenile polyposis of infancy (JPI)
- Generalized juvenile polyposis (colonic only or anywhere in GI tract)
JPS carries an increased risk of GI malignancy, with a lifetime risk of 38% to 68% (including patients who were participating in a JPS surveillance programme and those diagnosed with JPS at the time of cancer diagnosis—see recommendation 9). The Johns Hopkins Polyposis Registry data identified a relative risk for colorectal cancer (CRC) of 34.0 in individuals with JPS, a mean age of diagnosis of 43.9 years, and a cumulative lifetime risk of 38.7% (8.). Similarly the St Mark's Hospital UK Polyposis registry described a cohort in which 8.3% of polyps in JPS contained mild/moderate dysplasia and 14% patients developed cancers (9.). Referral bias may be a factor in these registry populations.
Genetics of Juvenile Polyposis Syndrome
Individuals with JPS may harbour germline mutations in the SMAD4 (18q21.1) or BMPR1A (10q23.2) genes (10., 11.). A gene mutation (SMAD4 or BMPR1A) will be identified in 40% to 60% of patients with JPS. Approximately 25% of patients have de novo mutations. Mutations in the tumour suppressor gene PTEN, also located on 10q22–23, 1Mb telomeric to BMPR1A, have been found in the germline of patients with CS, another polyposis syndrome with hamartomatous polyps (12.).
Germline missense mutations in ENG were identified in 2 of 14 young patients with JPS in whom there was no identified mutation in the SMAD4 and BMPR1A genes (13., 14.). Further data are required to determine whether there is an association of ENG mutations and JPS.
Genotype-phenotype correlations are inconsistent, with variable of age at presentation and number of polyps even in the same family with JPS, still some correlations have been established, for example, JPS patients with SMAD4 mutations are associated with more aggressive gastric polyps and appear to have a higher risk for gastric cancer (Table 2) (15.).
SMAD4—Hereditary Hemorrhagic Telangiectasia
JPS patients with SMAD4 mutation may have features of hereditary hemorrhagic telangiectasia. HHT is an autosomal dominant disorder of vascular dysplasia characterized by mucocutaneous telangiectases and organ arteriovenous malformations (AVMs) mainly affecting the lungs, liver, and brain. Telangiectases and AVMs are prone to bleeding, leading to chronic epistaxis and/or GI bleeding. Pulmonary and brain AVM's have the potential for life-threatening haemorrhage. HHT susceptibility genes encode proteins in the transforming growth factor β pathway, to which pathway SMAD4 and BMPR1A belong. A novel juvenile polyposis-HHT overlap syndrome has been identified in patients with juvenile polyposis due to SMAD4 mutation (16.). A cohort of 41 juvenile polyposis families found that nearly all juvenile polyposis SMAD4 patients have the overlap syndrome and concluded that systematic HHT screening is recommended for all JPS patients with SMAD4 mutations (16.) (see recommendation 4).
BMPR1A With PTEN
BMPR1A is located in the same chromosomal region as PTEN and larger deletions involving both genes have been reported. Children with deletions in both BMPR1A and PTEN genes may present with JPI. JPI is a distinct, aggressive subtype of JPS characterized by severe GI symptoms, including diarrhoea, intestinal bleeding, rectal prolapse, protein losing enteropathy and increased risk of intussusception resulting in high infant mortality (17.). JPI patients have been reported with congenital abnormalities including macrocephaly and generalized hypotonia. The rarity and variability of the JPI clinical presentation have made genotype phenotype correlation challenging (18.), and consensus surveillance recommendations cannot be reached (see recommendation 5).
Solitary Juvenile Polyps
Solitary JPs are the most common type of polyp in children comprising >90% of polyp cases, frequently presenting with rectal bleeding during early childhood. Solitary polyps often are located in the left colon, but as many as one third are located proximal to the splenic flexure hence the need for complete colonoscopy. The identification of multiple polyps would lead to the diagnosis of JPS (19.). Patients with solitary JPs may have >1 JP (but ⩽5 polyps)—either on initial presentation or subsequently in follow-up. Although there are few reports of adenomatous changes and only 1 report of adenocarcinoma in situ (19.) arising in a solitary JP, high-grade dysplasia is encountered rarely. There is no robust data to quantify the risk of malignant transformation, the risk is assumed to be extremely low (20.).
Q1: At what age should genetic testing be undertaken in at risk children?
Recommendation 1:
Routine predictive genetic testing for paediatric patients at risk of developing JPS should start at 12 to 15 years of age. Children who develop rectal bleeding earlier than this age should undergo colonoscopy and then proceed to genetic testing if polyps are identified.
Weak recommendation, very low quality of evidence.
Consensus agreement 100%
JPS is inherited in an autosomal dominant manner, with a 50% chance of inheriting the condition from an affected parent. No formal evidence-based guidelines exist on when to test the at risk child. Genetic testing in children for an inherited polyposis syndrome in which the primary morbidity, GI cancer, occurs many years later presents an ethical challenge for paediatric gastroenterologists (21.). This underscores the need for genetic counselling before presymptomatic testing in JPS as in all polyposis syndromes.
Predictive Testing a Child in an Affected Family
Predictive genetic testing can only be performed where the family mutation has been identified in the affected parent or affected sibling. If no pathogenic gene mutation has been identified in an affected parent or affected sibling, then the child cannot undergo predictive genetic testing and will require endoscopic screening (22.) (Fig. 1).
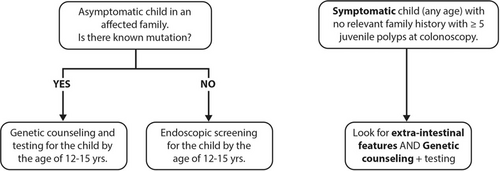
Genetic testing in JPS. When to test? JPS = juvenile polyposis syndrome.
There are case reports and published case series of adenomatous changes within JPs diagnosed in children as young as 4 years of age (20.). This does not affect the age of screening insofar as CRC is not documented in children younger than 20 years (8.). In a multicentre descriptive study out of 71 colonoscopies in 36 patients (mean age 7.35 ± 4 years), none of the 366 polyps removed demonstrated malignant change (23.). With the earliest age of onset of CRC described after the age of 20 years, there is no value in performing genetic predictive testing before the child can understand the genetic counselling and its implications. Predictive testing can be delayed until the age of 12 to 15 years, unless it is necessary to investigate a potentially affected individual for a noncolonic manifestation of JPS (Table 1). At risk children who present with rectal bleeding before the age of 12 years should undergo colonoscopy, whatever their age.
Genetic Testing in a Child With Multiple Colonic Polyps (≥5)
Children and adolescents with or without a relevant family history identified to have ≥5 JPs at colonoscopy, should be assessed for the extracolonic features of a JPS (Table 1) and subsequently referred for genetic counselling and genetic testing. A gene mutation might only be identified in 40% to 60% of patients with JPS', and if no pathogenic SMAD4 or BMPR1A variant is found then a multigene panel that includes PTEN is recommended.
Q2. What age should colonoscopic surveillance commence in children at risk or identified to have JPS
Recommendation 2:
Colonoscopic surveillance should commence from age 12 to 15 years, or earlier if symptomatic. Once polyps (>10 mm) are detected they should be removed and colonoscopy repeated annually until polyps >10 mm have been resected, then repeated every 1 to 5 years.
Weak recommendation, very low quality of evidence.
Consensus agreement 100%
Colonoscopic surveillance in JPS is necessary not only to prevent polyp-related morbidity (bleeding, anaemia, or abdominal pain) but also cancer prevention, as JPS polyps can undergo dysplastic and malignant transformation. When polyps are identified they should be removed to avoid bleeding and anaemia. At polypectomy, the polyp should be retrieved and sent for histological assessment.
For asymptomatic individuals with an SMAD4 or BMPR1A pathogenic variant identified by molecular genetic testing, individuals with a clinical diagnosis of JPS, or individuals with a family history of JPS whose molecular genetic test results were uninformative, colonoscopic surveillance can be delayed until age 12 to 15 years. Symptomatic children should undergo colonoscopy at any age (Table 2).
Ideally, colonoscopy should aim to remove all polyps >10 mm, and repeated annually until polyps of this size have been resected.
There are no data upon which decisions can be made as to the most appropriate interval for colonoscopy surveillance in JPS. A personalized approach with the surveillance interval (up to every 5 years) based upon individual colonic phenotype is the most appropriate approach (24., 25.).
3. What is the recommended age to commence gastroscopy in children with presumed or confirmed JPS?
Recommendation 3:
Surveillance of the upper GI tract in affected or at-risk JPS patients is not required in childhood or teenage years, unless there is unexplained anaemia or upper GI symptoms.
Weak recommendation, very low quality of evidence.
Consensus agreement 88%
There is a paucity of data evaluating the upper tract in JPS. The cancer risk in JPS is believed to arise from adenomatous tissue within the JP. Adult case series report the incidence of gastric polyps between 65% and 83% and duodenal polyps between 14% and 33% (26.). The reported lifetime risk of gastric carcinoma is between 10% and 25% for those who have gastric polyps (27.). Because of the limited data, it is difficult to determine the exact lifetime risk of gastric cancer in JPS.
There is a single case report of gastric malignancy reported in a JPS patient younger than 40 years of age who died 8 decades ago (28.). There were 2 gastric cancers in a cohort of 44 patients with JPS (median age 56 years) (9.). Three out of 5 patients who underwent gastroscopy younger than 15 years of age with gastric polyposis had no dysplasia (26.). In another series, 3 out of 18 patients with either SMAD4 or BMPR1A mutation who underwent gastroscopy had documented gastric polyps (28.) with a median age of upper GI carcinoma was 58 years. The risk of severe gastric polyposis and gastric cancer phenotype appears to be increased in patients with SMAD4 mutations (29.).
Current published guidelines for the age at which to start upper GI surveillance, ranges from 12 years (30.) to 25 years of age (31.).
Because of a paucity of published data, the spectrum of SMAD4 JPS upper tract findings is not well understood. In patients with confirmed or suspected SMAD4 mutation it is prudent to assess the upper tract in the later teenage years in those who are asymptomatic. In patients without SMAD4 mutation, according to the existing data, gastroscopy in childhood and teenage years is not indicated unless the patient has relevant upper GI symptoms or anaemia not explained by colonic polyps alone. As part of personalized polyposis care consideration of evaluation of the small bowel can be considered in patients with a severe gastric polyposis phenotype. If asymptomatic, gastroscopy is not required until adulthood.
Q4 What are the additional investigations indicated in a paediatric patient with SMAD4 mutation?
Recommendation 4:
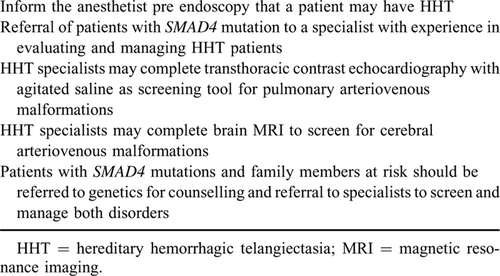
Paediatric patients with SMAD4 mutation should be evaluated for HHT including screening and preventative treatment for cerebral and pulmonary AVMs.
Strong recommendation, very low quality of evidence.
Consensus agreement 100%
The coexistence of JPS and HHT has been reported early in the 1980s (32.). Many patients with JPS due to SMAD4 mutation have features of HHT (epistaxis, telengiectases, clubbing, and AVM) (33.). Features of HHT may appear in childhood. Thoracic aortic disease and mitral valve dysfunction have been also reported in few SMAD4 positive JPS patients (9., 34.). As more JPS cases with SMAD4 mutations are characterized we will develop a better understanding of the associated vascular features.
Children with HHT may lack the typical clinical features of HHT such as telangiectasia or epistaxis, but are at risk of pulmonary and cerebral AVMs which can lead to life-threatening complications (35.). Clubbing has been reported in the combined JPS-HHT syndrome, a physical finding reported in approximately 10% of patients with pulmonary AVM (pAVM) (36.). pAVMs are often asymptomatic, but patients are at risk for secondary neurologic or hemorrhagic complications. Referral of patients with SMAD4 mutation to a specialist with experience in evaluating and managing HHT patients is recommended. Consensus guidelines for HHT recommend screening as soon as the diagnosis is made (37., 38.).
Screening for pAVMs is best completed by an expert in the care of patients with HHT. Transthoracic echocardiography is a sensitive screening test in children with suspected HHT and can be used as initial screening tool in the paediatric HHT population (39.). Screening should be performed at the time of initial clinical evaluation of HHT.
According to international guidelines, routine screening for cerebral AVMs is recommended with brain magnetic resonance imaging at diagnosis (38.). JPS patients found to harbour SMAD4 mutations and family members should be counselled by HHT specialist and genetic counselling and referred for screening related to both disorders as early testing will have relevance towards their management of the patient (Table 3).
Patients and families with suspected HHT should be referred to HHT centres of excellence, a useful resource can be found at (https://curehht.org/understanding-hht/get-support/hht-treatment-centers).
Q5 What are the additional investigations indicated in a child or adolescent with gene mutation located at BMPR1A.
Recommendation 5:
In JPS patients with an isolated BMPR1A gene mutation, there are no additional investigations required beyond the endoscopic procedures described above.
Children with BMPR1A mutation and early onset polyposis and/or a severe phenotype and/or extraintestinal manifestations should be evaluated for PTEN mutation.
Weak recommendation, very low quality of evidence.
Consensus agreement 100%
In JPS patients with an isolated BMPR1A gene mutation, there are no additional investigations required beyond the endoscopic procedures described above.
PHTS encompasses 4 major clinically distinct syndromes associated with germline mutations in the tumour suppressor PTEN. Mutations of the PTEN gene localized at 10q22–23 are associated with CS, and the lesser known Bannayan-Riley-Ruvalcaba syndrome (40.) associated with macrocephaly, dysmorphism, and developmental delay. These conditions, known as the “PTEN-hamartoma syndrome” may present with juvenile or hamartomatous polyps.
BMPR1A is located in the same chromosomal region as PTEN and deletions involving both genes have been reported. There are numerous case reports suggesting that if the mutation (microdeletion) is found in both genes, these patients present with a severe form of JPS with onset in early childhood (JPI) or may have symptoms of both CS and JPS with phenotypic diversity (14., 41.). As a correct clinical and molecular diagnosis has important consequences for appropriate clinical management, patients with early onset polyposis and/or severe phenotype and/or extraintestinal manifestations (macrocephaly, mental retardation) should be referred to geneticists for more extensive analysis of the BMPR1A and PTEN genes. Children with BMPR1A and PTEN mutations have an increased risk of severe intestinal polyposis (42., 43.) and may carry a greater risk of GI malignancy, although the absolute risk cannot be defined due to the small numbers reported in the literature, which is, in turn, almost certainly biased towards more severe phenotypes. Patients with both mutations of PTEN and BMPR1A may require frequent or annual gastroscopy, colonoscopy, and small bowel evaluation from early childhood. Moreover, they are at risk for cardiac, endocrine, GI, and neurodevelopmental abnormalities (41.).
Q6 Do patients with a single JP require repeat colonoscopy?
Recommendation 6:
In a child with a single JP, a repeat colonoscopy is not routinely required.
Weak recommendation, very low quality of evidence.
Consensus agreement 100%
Solitary JP represents 70% to 80% of polyps found in children (44.). A few of those children harbour a risk to form more polyps over time. Recurrence of new solitary JP varies from 4% to 17% (19., 24.), but the risk of developing malignancy in childhood remains low or not at all (20., 45.) (Table 4). There are case reports of neoplastic change in children with solitary JPs and yet, single JPs are generally regarded as benign with no or minimal risk of future malignancy (46., 47.). After endoscopic solitary juvenile polypectomy, provided there are no suspicious histologic features, and family history of GI polyps, cancer, or HHT, the child can be discharged and the parents instructed to return if symptoms arise.
Fox et al (24.) published data, which reveals that solitary polyps in children recur in approximately 17% of cases and neoplasia (adenomatous foci) occurs in 3.9%, suggesting that current clinical practice may be inadequate. Still, further studies are needed to estimate the real risk of recurrence and neoplasia in solitary JP. To date, no surrogate markers of solitary JPs in children is available for future surveillance to prevent future malignancy and no evidence-based practice guidelines exist for patients with one or a few JPs, who do not fulfil the criteria for JPS.
When 2 or 3 JPs are found during the initial colonoscopy, surveillance colonoscopy is a valid option and whilst it is uncertain if the child may subsequently develop JPS, repeating the colonoscopy after 5 years, or earlier if rectal bleeding returns, seems appropriate.
Q7 Should parents and siblings of an affected child undergo colonoscopic surveillance?
Recommendation 7:
If a specific gene mutation has been detected in a child, then genetic testing should be offered to all first-degree family members. If no specific gene mutation was detected, then first-degree relatives should be referred for screening colonoscopy at the age of 12 to 15 years.
Weak recommendation, very low quality of evidence.
Consensus agreement 100%
The diagnosis of JPS has implications for first-degree relatives; therefore, genetic counselling plays a crucial role in the management of kindred with JPS. Genetic counselling within a multidisciplinary clinic can offer support and education for this complex diagnosis that has implications for the entire family.
As many as 75% of individuals diagnosed with JPS have an affected parent, although the family history of some individuals diagnosed with JPS may appear to be negative, while the others (25%) have no previous history of polyps in the family and the mutation could arise de novo (7.).
If a specific gene mutation (SMAD4 or BMPR1A) has been detected in a child, then presymptomatic genetic testing should be offered to all first-degree family members to determine future surveillance strategies and to allow for early diagnosis and treatment (48.). Predictive genetic testing can be offered to at-risk siblings from age 12 years or earlier if symptoms develop.
If no specific gene mutation was detected in the child, then asymptomatic first-degree relatives should be considered for screening by colonoscopy, starting at 12 to 15 years of age, given that the cancer risk in young age is almost nonexistent and that benign colonic JPs, often present with rectal bleeding (6.). Continued surveillance of siblings after first negative colonoscopy may be required since polyps may appear later in life but prospective evidenced-based data are lacking. If the pathogenic gene mutation found in the child cannot be detected in either parent, then the risk to the sibs can be assumed to be population risk (7.).
Q8: Is there a role for chemoprevention therapies in juvenile polyposis?
Recommendation 8:
There is no role for chemoprevention in JPS.
Weak recommendation, very low quality of evidence.
Consensus agreement 100%
To date, there have been no chemoprevention trials in JPS in adults or children and therefore no therapy can be recommended in JPS. If studies are performed, robust, clinically meaningful endpoints will be required.
Cyclooxygenase-2 (COX-2) inhibitors have been considered in polyposis syndromes given the broad expression of COX-2 in adenomas in familial adenomatous polyposis (FAP) (49.) and in Peutz-Jeghers polyps and in hereditary mixed polyposis syndrome (50., 51.). There are reports describing increased COX-2 expression in JPS related to both polyp size and presence of dysplasia (52., 53.). COX-2 expression was higher in JPS with BMPR1A mutations than in JPS without mutations. There exists a theoretical potential benefit in the use of selective COX-2 inhibitors in JPS, but to date, there are no trials demonstrating efficacy and there is no proven role for the use of COX-2, or any other chemopreventive agent in JPS.
Q9 What is the cancer risk in children and young adults with JPS?
Recommendation 9:
Adult patients with JPS, have an increased risk of cancer especially in the GI tract. In children younger than 18 years, GI cancer is rare and limited to case reports; therefore, surveillance is not needed before 18 year.
Weak recommendation, very low quality of evidence.
Consensus agreement 100%
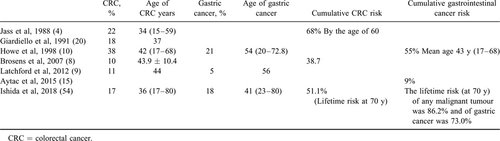
JPS is reported to be associated with an increased risk of malignancies; mainly colorectal and gastric cancer in adults (Table 5). In the earliest descriptions of JPS, Jass et al (4.) reported the pathological findings of 80 patients with JPS; 18 patients (22%) have developed CRC at a mean age of 34 years (range 15–59). Subsequently, Giardiello et al (20.) reported an 18% risk of CRC in a retrospective study; the risk was higher in familial (40%) than in nonfamilial (5%) JPS; CRC occurred at a mean age of 37 years. Their study reported 4 cases of JPS that had neoplasia (adenomatous epithelium and/or tubulovillous adenomas) in young children who were 3, 4, and 7 years old. Data from the Johns Hopkins Polyposis Registry were updated in 2007 describing 8 colon cancers in 84 JPS patients (8.). The absolute risk of CRC was 38.7%, whereas the relative risk of CRC in JPS compared to the US general population was 34 (males 30, females 43.7). The mean age at diagnosis was 43.9 years (standard deviation 10.4 years). A later British study reported that 14% of 44 JPS patients developed cancer at a mean age of 44 years for CRC and 56 years for gastric cancer, respectively (9.). Recent data from Japan published by Ishida et al reported a lifetime cancer risk of 86.2% for any malignant tumour. The lifetime risk of gastric cancer was 73.0% (the risk of gastric cancer in Japan is higher than that in western populations). One third of the patients in this study had juvenile polyposis limited to the stomach. The lifetime risk of CRC was 51.1%. The risk of gastric and colonic cancer was dependent on the distribution of the polyps in the GI tract. The cumulative risk was significantly lower in the patients with JPs limited to the colon compared to patients with a more widespread GI phenotype including the stomach and colorectum (54.).
A study differentiating the genetic findings of JPS and HHT, showed the risk of CRC being higher in JPS patients with SMAD4 mutations; 3 CRC occurred in 14 SMAD4 patients at a mean age of 34 years (36.). Indeed, the risk of cancer may vary between different genotypes, for example, gastric cancers were reported more likely for patients harbouring SMAD4 and not BMPR1A mutations (9., 29.).
Gastric polyposis is more prevalent in SMAD4 than in BMPR1A JPS, whereas gastric cancers developed only in SMAD4 patients (23., 55.) and advanced upper GI disease was more likely in those with had SMAD4 mutations. From the paediatric perspective, it is harder to quantify the risk of cancer during childhood (24., 45.). Fox et al reported a cohort data of 257 children with 1 or more JPs. Approximately 4% of children with JPs had neoplasia, with 9 children having low-grade dysplasia and one 11.8-year-old boy with adenocarcinoma. Each of these patients with neoplasia had >5 polyps detected during the initial colonoscopy with a range of 7 to >100 (24.). Despite this report, malignancy in JPS in children and adolescents younger than 18 years is exceptionally rare.
Finally, in JPI with deletions encompassing PTEN and BMPR1A, the risk of malignancies will be associated with the deletion of PTEN. This risk should be similar to patients with CS or Bannayan-Riley-Ruvalcaba syndrome, which is principally about non-GI malignancies in adulthood, for example, breast or thyroid cancer. In these patients, advice should be sought from a geneticist. Noncolonic malignancies have been reported in children with loss of BMPR1A and PTEN due to chromosomal microdeletion, including bilateral ovarian mucinous cystadenoma diagnosed at 14 years (56.).
The interpretation of all literature requires critical appraisal to assess for potential biases. Data collected from patients monitored under a polyposis surveillance programme, enrolled in registries, or followed in tertiary clinic settings may not be generalizable to other populations. The polyposis phenotype may vary among populations. For example, the study from Japan reported a high proportion of gastric polyps limited to the stomach. Detection bias can be a factor if cases are identified due to a diagnosis of cancer. Ascertainment bias can be a factor. Different methodologies can affect the results. For example, estimates of CRC risk will be affected if patients undergo partial or total colectomy.
Question 10. What is the role of colectomy in patients with JPS?
Recommendation 10:
Colectomy should be discussed only in patients in whom the burden of polyps is not manageable by polypectomy alone, and/or leading to uncontrollable anaemia or hypoalbuminemia from colonic polyposis.
Weak recommendation, very low quality of evidence.
Consensus agreement 100%
There are no published paediatric or adult papers focused on the indications for colectomy in JPS. Colectomy should be reserved for those patients in whom the burden of disease cannot be managed by polypectomy alone. Indications for colectomy in JPS include excessive polyp burden that is too difficult or perilous to manage endoscopically, and persistent blood loss resulting in persistent anaemia, or hypoalbuminaemia (57.). As described above, dysplasia has been reported in JPS, but malignancy is exceptionally rare. Therefore colectomy should not be recommended on the presence of dysplasia in 1 polyp. The decision to proceed to colectomy should be discussed with gastroenterologists and surgeons at a centre specializing in hereditary polyposis syndromes in children.
FUTURE DIRECTIONS
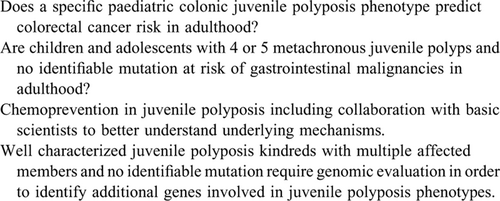
Future aims include a more uniform approach among paediatric gastroenterologists globally in the management of JPS patients. Prospective monitoring of paediatric JPS patients including collection of data in a systematic fashion will be crucial to advance the field. Juvenile polyposis is an area requiring more research (Table 6). International collaboration and ultimately consortia are proposed to monitor patients prospectively to lead to a more comprehensive understanding of juvenile polyposis conditions. Collaboration with basic scientists is necessary to better understand underlying mechanisms that lead to the development of juvenile polyposis phenotypes. International Consortia exist, which could expedite collaboration with adult gastroenterologists. Over time these strategies will lead to precise data delineating lifetime GI cancer risk in JPS patients (Table 7).
Well characterized juvenile polyposis kindreds with multiple affected members and no identifiable mutation require focused genomic evaluation to identify additional genes involved in juvenile polyposis phenotypes. All of these efforts will result in better understanding of genotype-phenotype correlations, accurate life time cancer risks, and role of chemoprevention in JPS.
SUMMARY OF RECOMMENDATIONS
- Routine predictive genetic testing for paediatric patients at risk of developing JPS should start at 12 to 15 years of age. Children that develop rectal bleeding earlier than this age should undergo colonoscopy and then proceed to genetic testing if polyps are identified. Weak recommendation, very low quality of evidence.
- Colonoscopic surveillance should commence from age 12 to 15 years, or earlier if symptomatic. Once polyps (>10 mm) are detected they should be removed and colonoscopy repeated annually until polyps >10 mm have been resected, then repeated every 1 to 5 years. Weak recommendation, very low quality of evidence.
- Surveillance of the upper GI tract in affected or at-risk JPS patients is not required in childhood or teenage years, unless there is unexplained anaemia or upper GI symptoms. Weak recommendation, very low quality of evidence.
- Paediatric patients with SMAD4 mutation should be evaluated for HHT including screening and preventative treatment for cerebral and pulmonary AVMs. Weak recommendation, very low quality of evidence.
- In JPS patients with an isolated BMPR1A gene mutation, there are no additional investigations required beyond the endoscopic procedures described above. Children with BMPR1A mutation and early onset polyposis and/or a severe phenotype and/or extraintestinal manifestations should be evaluated for PTEN mutation. Weak recommendation, very low quality of evidence.
- In a child with a single JP, a repeat colonoscopy is not routinely required. Weak recommendation, very low quality of evidence.
- If a specific gene mutation has been detected in a child, then genetic testing should be offered to all first-degree family members. If no specific gene mutation was detected, then first-degree relatives should be referred for screening colonoscopy at the age of 12 to 15 years. Weak recommendation, very low quality of evidence.
- There is no role for chemoprevention in JPS. Weak recommendation, very low quality of evidence.
- Adult patients with JPS, have an increased risk of cancer especially in the GI tract. In children younger than 18 years, GI cancer is rare and limited to case reports therefore surveillance is not needed before 18 year. Weak recommendation, very low quality of evidence.
- Colectomy should be discussed only in patients in whom the burden of polyps is not manageable by polypectomy alone and/or leading to uncontrollable anaemia or hypoalbuminemia from colonic polyposis. Weak recommendation, very low quality of evidence.
DISCLAIMER
ESPGHAN is not responsible for the practices of physicians and provides guidelines and position papers as indicators of best practice only. Diagnosis and treatment is at the discretion of physicians.