Growth of Indonesian Infants Compared With World Health Organization Growth Standards
www.clinicaltrials.gov registration number: NCT01721512.
This study received a grant and commercial infant formula from Fonterra Brands (Singapore) Pte Ltd.
The authors report no conflicts of interest.
ABSTRACT
Objectives:
The ability of the World Health Organization (WHO) growth standards to represent the growth of South East Asian infants has been questioned. The aim of this study was to provide contemporary data on the growth of Indonesian breast-fed and formula-fed infants, compared with the WHO growth standards.
Methods:
A prospective cohort study of 160 normal healthy infants was undertaken in a suburban area of South Jakarta, Indonesia. Infants from 2 to 6 weeks of age were recruited, and they consumed exclusively either breast milk or infant formula for at least 6 months, with follow-up until 12 months of age.
Results:
Overall, the infants in the present study were lighter (weight-for-age), were shorter (length-for-age), and had smaller head circumferences (head circumference-for-age) than the average WHO Growth Reference Study population but were of similar proportion (weight-for-length). Compared with the WHO Growth Reference Study, the z scores for weight-for-age, length-for-age, and head circumference-for-age in the Indonesian children fell from birth to 6 weeks of age and then increased until 3 months of age in both the breast-fed and the formula-fed infants. At 6 weeks of age, the weight-for-age z scores fell below −2 standard deviations for 16 (20.5%) breast-fed and 40 (51.3%) formula-fed infants, and the length-for-age z scores fell below −2 standard deviations for 31 (39.7%) breast-fed and 41 (52.6%) formula-fed infants.
Conclusion:
The WHO growth standards do not reflect the growth of the present cohort of Indonesian infants and may overestimate the levels of underweight and stunted children.
In 2006, the World Health Organization (WHO) launched new growth standards for infants and children 1. These standards aimed to be internationally representative and to depict how children should grow when free from disease and fed according to recommendations 2. As such, the WHO Growth Reference Study (WGRS) selected singleton, term infants without health, environmental, or economic constraints on growth from study sites in 6 countries (Brazil, Ghana, India, Norway, Oman, and the United States) 3.
By April 2011, at least 125 countries, representing 75% of the world's under-5 population, had adopted the WHO growth standards. The ability of these standards to represent individual countries and populations has been questioned 4. Of note, no data for East Asian infants, who constitute one-fifth of the global population, were included 5. For example, it has been shown that ethnic Chinese infants living in Hong Kong are shorter at 36 months 5 and that Japanese breast-fed infants are significantly shorter and lighter throughout almost the first 24 months compared with the WHO growth standards 6.
The aim of this paper was to analyze growth data from a feeding study 7 and to compare the growth of Indonesian breast-fed and formula-fed infants with the WHO growth standards.
METHODS
Study Design
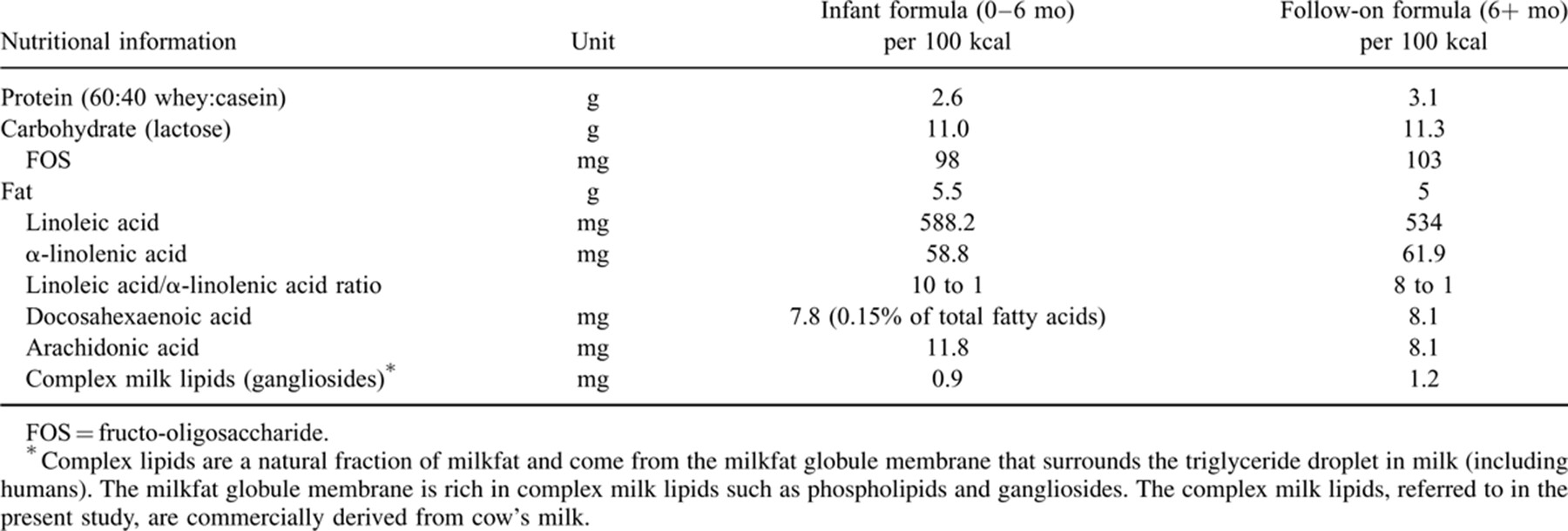
A prospective cohort study of 160 healthy, full-term, singleton infants, recruited at 2–6 weeks of age, was undertaken in a suburban area of South Jakarta from September 2010 to November 2011. Breast-fed infants were eligible if their mothers intended to exclusively breast feed until at least 6 months of age. Formula-fed infants were included if they had been exclusively formula fed before enrolment, and their mothers consented to offering the prescribed cow's milk infant formula until 6 months of age and then follow-on formula until 12 months of age. (Anmum infant formulae Step 1 and Step 2 [Table 1, supplied by Fonterra Brands (Singapore) Pte Ltd] contain whole protein, probiotic bacteria, prebiotic, complex milk lipids [source of gangliosides], and long-chain polyunsaturated fatty acids from plant and algal sources.) Further information on the study is detailed in the associated publication resulting from the present research 7.
Ethical approval was obtained from the Ethics Committee of the Faculty of Medicine Universitas Indonesia. Informed consent to participate in the study was obtained from parents at recruitment.
Data Collection
The primary outcome of the study was growth; secondary outcomes included gastrointestinal tolerance, developmental progress, and general health.
At enrolment (2–6 weeks), birth weight, feeding history, family history, stool frequency, and characteristics were recorded and a physical examination was undertaken. The study visits (n = 8) were conducted at 6 weeks and 2, 3, 4, 6, 8, 10, and 12 months of age, with a tolerance of 10% around these targets.
Growth
Infants had their weight, length, and head circumference measured at each study visit. Infants were weighed naked in triplicate on the same calibrated electronic scales to the nearest 10 g. The recumbent length was measured in triplicate using a Harpenden Neonatometer and the head circumference was measured in duplicate at the largest occipitofrontal circumference, using a nonstretchable tape, to the nearest 1 mm.
Secondary Outcomes
Up to the fourth month, parents were asked to recall sleeping, waking, and crying patterns of their infant during the previous week. Stool frequency and stool consistency (according to the Bristol Stool Chart 8) were reported by parents for the 3 days before each visit up to the sixth month, and the use of medications was recorded at each visit. The Ages and Stages Questionnaire 2 8 was used to assess infant development at 3, 6, 10, and 12 months of age. Adverse events were documented and were rated for severity at each study visit.
Statistical Analysis
Sixty-four infants per group were required for a difference in weight gain between groups of 0.5 standard deviation with a power of 80% and a significance of 5%. Eighty infants were recruited for each group to allow for up to 25% dropout. Statistical analysis was on an intention to treat basis. We selected a sample of Indonesian infants with the aim of describing a population of breast-fed and formula-fed infants and compared them to the WHO standards, so including all available data provided the most representative comparison.
All available data were included in the analyses. z scores for weight-for-age, length-for-age, weight-for-length, and head circumference-for-age were calculated using the WHO SAS macro (http://www.who.int/childgrowth/software/en WHO Anthro version 3.2.2, January 2011). Individual z scores were analyzed using a mixed models approach to repeated measures ANOVA (Proc Mixed, SAS 9.3; SAS Institute, Cary, NC), followed by Turkey t test. P ≤ 0.05 was declared to be statistically significant.
RESULTS
Participants
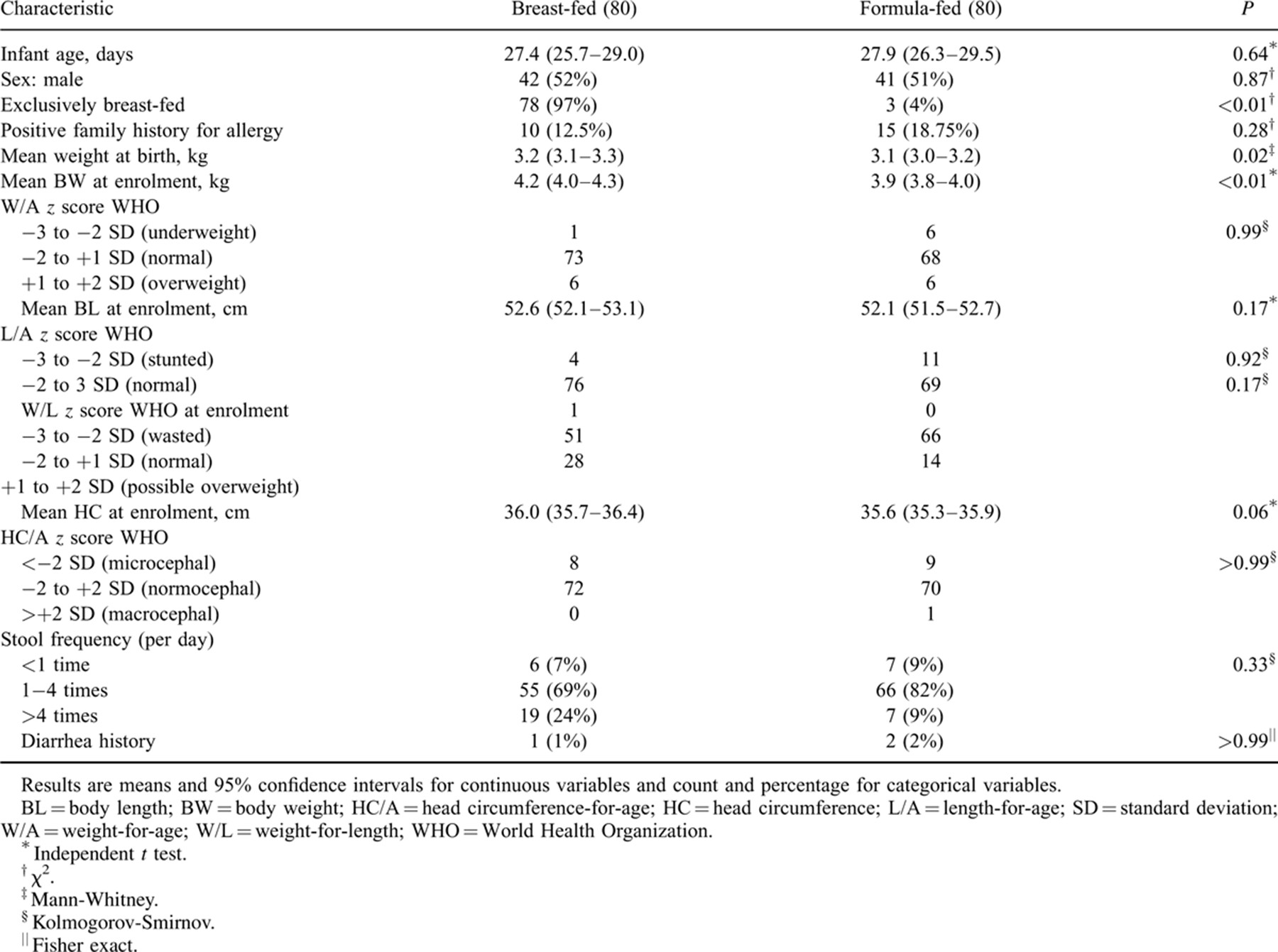
The infants were mostly from middle-class families (per capita income US$ 1026–12,475). There was no significant difference between breast-fed and formula-fed infants with respect to age, sex, body length, head circumference, stool frequency, or diarrhea history at enrolment (Table 2). Weights at birth and enrolment were significantly higher in the breast-fed group (P = 0.022 and P = 0.003). At 4 and 8 months, 17 and 21 infants had been withdrawn, respectively. Twenty-eight infants (17.5%) did not complete the 12-month follow-up because of either relocation away from the study area (10 formula-fed infants and 7 breast-fed infants) or noncompliance (9 formula-fed infants and 2 breast-fed infants).
Growth
The infants in the present study were lighter, were shorter, and had smaller head circumferences when compared with the WGRS population, but were of similar proportion. Fig. 1 shows the change in z scores during time by study group for each of the z score parameters. With the exception of birth weight for the breast-fed group, all the z scores for weight-for-age, length-for-age, and head circumference-for-age fell below 0. The z scores for weight-for-length fell between −1 and 1.
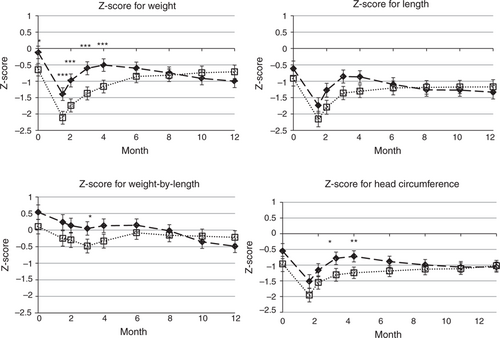
World Health Organization growth standard z scores for breast-fed and formula-fed infants. Results are least squares means and 95% confidence intervals. *P < 0.05, **P < 0.01, ***P < 0.001; -- breast-fed, □ formula-fed.
The breast-fed group remained significantly heavier (weight-for-age) than the formula-fed group until 4 months, but the formula-fed group had caught up by 6 months and there was no significant difference between the weight-for-age z scores between feeding groups for the remainder of the study. The differences were smaller for the other z score parameters.
The infants in the present study showed a different pattern of growth compared with the WGRS population. The z scores for weight-for-age, length-for-age, and head circumference-for-age fell from birth to 6 weeks and then showed an increase until 3 months in both the breast-fed and the formula-fed infants. The z scores for the formula-fed infants then continued to increase but at a more modest rate, particularly after 6 months. In contrast, the z scores for the breast-fed infants stabilized between 3 and 4 months and then showed a modest decrease in all 3 z score parameters. This suggests that these infants grew more slowly than the WGRS population in the first 6 weeks but then grew faster until at least 3 months of age.
Gastrointestinal Tolerance, Development, and General Health
No clinically significant differences between the 2 study groups in stool frequency (1–2 times per day during the first 6 months) and stool characteristics were reported.
No significant differences between the groups were observed in terms of frequency of daytime sleeps or night-time waking-up, duration of sleep or crying in the day or evening time, or time taken to settle. At 4 months, the formula-fed infants slept longer (45 minutes, P = 0.030) and cried slightly longer at night (2.5 minutes, P = 0.037) than the breast-fed infants.
Most infants in both the study groups had normal development patterns throughout the study period. The groups did not differ for overall development scores or scores of problem solving or gross motor function at any age. The only differences in Ages and Stages Questionnaire 2 scores between the groups were that the communication skill score was higher for the infants in the formula-fed group at 3 months (P = 0.026) and that the personal social score was significantly higher for infants in the breast-fed group at 6 months (P = 0.006).
One infant within the infant formula group suffered cow's milk allergy as an adverse event and 1 infant in the breast-fed group had aspirational pneumonia.
Overall, there was no difference between the breast-fed and formula-fed groups in the occurrence or severity of adverse events, respiratory problems, gastrointestinal problems, and allergy/skin problems during the study.
DISCUSSION
Despite including a group of infants without signs or symptoms of disease and of normal size at birth, we found that the present cohort of Indonesian children was smaller than the WGRS cohort in terms of weight-for-age, length-for-age, and head circumference-for-age but was of similar proportion, as shown by a comparable weight-for-length. As suggested by Hui et al 5, this may reflect differences in genetic growth potential or intergenerational epigenetic restrictions on growth potential in east Asian populations compared with the WGRS populations.
It has been noted that the weight-for-age percentiles of the WHO growth standards are higher than previously used growth references from birth to 6 months of age 4; some authors have suggested that this may convince breast-feeding mothers that their milk supply is insufficient to allow their child to thrive and therefore to induce them to inappropriately introduce “top-up” foods or breast-milk substitutes or even to discontinue breast-feeding completely 9, 10. The Indonesian women included in the present cohort, however, continued to breast feed, and the breast-fed infants had higher weight-for-age z scores than the formula-fed group from enrolment to 4 months, although weight-for-age for the breast-fed infants remained below the expected WHO Standard. This may support the suggestion that the high weight-for-age percentiles before 6 months in the WHO Standard may result from the large selective dropout or exclusion of infants who did not comply with the WGRS breast-feeding requirements 4.
Using the WHO Global Database on Child Growth and Malnutrition 11z score cutoffs, the present cohort of infants would not be classified as wasted (low weight-for-length), but a significant proportion would be classified as moderately or severely underweight (low weight-for-age) and/or moderately or severely stunted (low length-for-age), which could suggest chronic undernutrition. In actual fact, the study design (midwife support and encouraging breast-feeding or the provision of infant formula to formula-fed infants) suggests that, at least until 6 months of age, these infants were likely to have received sufficient nutrition. These results suggest that the WHO growth standards may overestimate the prevalence of chronic undernutrition in east Asian populations, which could have the effect of stretching already limited resources in public health. It has been suggested that alternative diagnostic criteria, such as different z score cutoffs, be used to assess levels of undernutrition in infants under 6 months 12.
CONCLUSIONS
The present study provides contemporary data on the growth of both breast-fed and formula-fed Indonesian infants. It reveals that the WHO growth standards do not reflect the growth of the present cohort of Indonesian infants and overestimate the levels of underweight and stunted children. On an individual level, this has the potential to cause concern and alter feeding behavior, whereas, on a population level, it may lead to public health resources being diverted from those most in need of them.
Acknowledgments
The authors thank P. Brown and B. Kuhn-Sherlock for technical assistance in manuscript writing and statistical analysis. The authors also express their gratitude to Dr Soedjatmiko and his team for providing development assessments, and to Drs M. Ashary, G. Fadiana, M. Albertina, and E. Renjana for daily trial support. Special thanks are also given to all of the infants and their families who participated in the study.