Inferior Outcomes of EU Versus US Patients Treated With CD19 CAR-T for Relapsed/Refractory Large B-cell Lymphoma: Association With Differences in Tumor Burden, Systemic Inflammation, Bridging Therapy Utilization, and CAR-T Product Use
VB, AP, and KR have contributed equally to this work.
MS and MDJ have contributed equally to this work.
Supplemental digital content is available for this article.
Graphical Abstract
Abstract
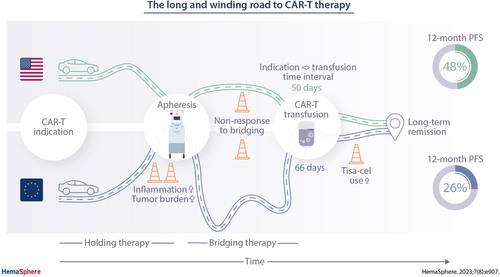
Real-world evidence suggests a trend toward inferior survival of patients receiving CD19 chimeric antigen receptor (CAR) T-cell therapy in Europe (EU) and with tisagenlecleucel. The underlying logistic, patient- and disease-related reasons for these discrepancies remain poorly understood. In this multicenter retrospective observational study, we studied the patient-individual journey from CAR-T indication to infusion, baseline features, and survival outcomes in 374 patients treated with tisagenlecleucel (tisa-cel) or axicabtagene-ciloleucel (axi-cel) in EU and the United States (US). Compared with US patients, EU patients had prolonged indication-to-infusion intervals (66 versus 50 d; P < 0.001) and more commonly received intermediary therapies (holding and/or bridging therapy, 94% in EU versus 74% in US; P < 0.001). Baseline lactate dehydrogenase (LDH) (median 321 versus 271 U/L; P = 0.02) and ferritin levels (675 versus 425 ng/mL; P = 0.004) were significantly elevated in the EU cohort. Overall, we observed inferior survival in EU patients (median progression-free survival [PFS] 3.1 versus 9.2 months in US; P < 0.001) and with tisa-cel (3.2 versus 9.2 months with axi-cel; P < 0.001). On multivariate Lasso modeling, nonresponse to bridging, elevated ferritin, and increased C-reactive protein represented independent risks for treatment failure. Weighing these variables into a patient-individual risk balancer (high risk [HR] balancer), we found higher levels in EU versus US and tisa-cel versus axi-cel cohorts. Notably, superior PFS with axi-cel was exclusively evident in patients at low risk for progression (according to the HR balancer), but not in high-risk patients. These data demonstrate that inferior survival outcomes in EU patients are associated with longer time-to-infusion intervals, higher tumor burden/LDH levels, increased systemic inflammatory markers, and CAR-T product use.
INTRODUCTION
Chimeric antigen receptor (CAR) T-cell therapy has demonstrated remarkable efficacy across a spectrum of advanced B-cell malignancies both in clinical trials and the real-world setting.1.-12. However, the therapy platform presents with a unique side effect spectrum, which typically includes cytokine-release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), but also prolonged cytopenias and infectious complications.13.-18. Furthermore, the individual patient journey of CAR-T delivery involves several key logistic steps, starting at indication (usually consented in a tumor board meeting) to leukapheresis, manufacturing, shipping, and re-infusion.19.
Real-world evidence for CAR-T therapy in the third-line setting has largely confirmed the results from the key registrational trials,6.-8. although a wide range of outcomes has been reported for different geographic regions or administered CAR T-cell products.9.,11. Several important features distinguish the real-world setting from clinical trials, such as (1) inclusion of patients that would not fulfill key study inclusion criteria, (2) differences in manufacturing times, and (3) the necessity of bridging therapy.
In addition, regional- and center-specific differences in referral patterns and nuances in the definition of eligibility may influence access to CAR-T therapy. For example, variability exists in the evaluation of nonresponse to chemoimmunotherapy before autologous stem cell transplantation (ASCT) or how stringently patients are selected or excluded from CAR-T treatment due to concomitant organ dysfunction. Waiting times for insurance authorization, leukapheresis, and manufacturing slots may also differ for regions and CAR-T products, and overall may contribute to differences in real-world survival outcomes.
Finally, the administration and nature of these intermediary therapies (applied between indication and infusion) remains heterogeneous and dependent on local standard-of-care practice. Patients may receive cytoreductive therapy between indication and leukapheresis, hereafter termed holding therapy. Additional bridging therapy, defined as therapy given during manufacturing between leukapheresis and infusion, may also be applied. While the use of bridging therapy was not allowed in the registrational trials of axicabtagene-ciloleucel,2.,20.,21. the overwhelming majority of patients receive bridging in the real-world setting (>80%)9. and its use is associated with inferior outcomes.9.,22.-24.
Ultimately, region-wide and product-specific differences in baseline and dynamic patient characteristics of known negative prognostic influence, including tumor burden and inflammation, may affect the outcomes of CD19 CAR-T therapy.25.,26. In this multicenter retrospective observational study, we therefore aimed to characterize differences in patient baseline features, intermediary therapy and CAR-T cell product use, and their respective relationship with post-CAR-T clinical outcomes.
METHODS
Study design, participants, and definitions
All 6 participating centers obtained independent institutional review board approval for this retrospective observational study. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines. Clinical data were extracted from the medical records and databases. A leniency period of ≤5 days was permitted for laboratory markers. Patients received standard-of-care axi-cel5. or tisa-cel17. according to the US Food and Drug Administration or European Medicines Agency approval for relapsed/refractory (R/R) large B-cell lymphoma (LBCL) in the third- or higher-line setting. The study time period was from January 2018 to August 2021 (data cutoff). Patients who underwent leukapheresis but subsequently did not receive infusion or patients receiving a second CAR T-cell treatment were excluded. However, the study did include patients who received out-of-specification CAR T-cell products on expanded access protocols.
Static and dynamic patient characteristics were assessed at several time points over the course of the therapy: (1) at indication (usually defined by a tumor board decision confirming eligibility for CAR-T therapy, or alternatively by first visit where informed consent for CAR-T was obtained); (2) at leukapheresis; and (3) immediately before lymphodepletion. CRS and ICANS were graded using the ASTCT consensus criteria.14. Nonrelapse mortality (NRM) was defined as death after cellular therapy without prior relapse or progression. Response was assessed locally according to the 2014 Lugano classification.27.
Statistical methods
Descriptive statistics are reported for all patients. To assess associations between categorical variables, Fisher exact test or χ2 test was used. For comparisons of continuous variables, the Mann-Whitney test was used. Kaplan-Meier estimates of progression-free survival (PFS) were calculated from the time of infusion to the date of progression, last follow-up or death. The log-rank test was calculated to evaluate differences between patient groups. Statistical analysis and data visualization was performed using GraphPad Prism (v9.0) or R Statistical Software (v4.1.0).
Univariate and multivariate modeling and high risk (HR) balancer/PRE balancer development
Univariate Cox regression analysis was performed to study the influence of static and dynamic patient characteristics on PFS. Covariates with a P-value ≤0.1 on univariate analysis were introduced into multivariable Lasso penalized regression models. Details on model generation are given in the Supplemental Digital Content methods. The identified variables were weighted by their respective Lasso coefficient to develop the high risk (HR) balancer and the PRE balancer (whereas the latter only included variables available at CAR-T indication), both balancers representing continuous scores for patient-specific risk classification. Finally, we developed confirmatory multivariable Lasso models, one including the HR balancer to identify other, HR balancer-independent variables associated with PFS, and one including potentially relevant interactions (HR_interaction).
RESULTS
European patients exhibit longer vein-to-vein intervals and a combination of adverse prognostic markers before CAR T-cell therapy
In this real-world cohort, the median age was 64 years (range, 19–85), median Eastern Cooperative Oncology Group (ECOG) performance status at indication was 1 (interquartile range [IQR], 0–1), and median international prognostic index (IPI) was 3 (IQR, 2–4) (Table 1). Patients received a median of 3 prior treatment lines (not counting holding and bridging), including 98 patients (26%) with a prior ASCT. Extranodal disease manifestations were noted in 265 patients (71%), including 34 patients (16%) with a history of, or active, central nervous system disease.
When studying region-specific differences in CAR-T logistics, we observed that patients treated in the Europe (EU) displayed markedly longer average indication-to-infusion times compared with their US counterparts (66 versus 50 d; P < 0.001) (Figure 1A and 1B; Suppl. Table 1). Notably, this was predominantly driven by a longer average time interval between leukapheresis and CAR-T infusion, the so-called vein-to-vein interval, in patients treated in the EU (49 versus 30 d; P < 0.001). Conversely, the average indication-to-apheresis time was slightly longer in the US-treated patients (20 versus 17 d). For demographic and laboratory features, we found that lactate dehydrogenase (LDH) levels were significantly higher in the European patients at indication (median 321 versus 271 U/L; P = 0.02), apheresis (median 327 versus 268 U/L; P = 0.007), and before lymphodepletion (median 302 versus 261 U/L; P = 0.04; Figure 1C; Suppl. Table 2). C-reactive protein (CRP) levels were comparable in both study cohorts (median 1.45 versus 1.29 mg/dl; P = 0.72). Ferritin was significantly elevated across all study time points in the EU patients, particularly at apheresis (median 734 versus 439 ng/mL; P = 0.004) and lymphodepletion (median 675 versus 425 ng/mL; P = 0.004).
| n | All Patients (n = 374) |
US/Moffitt (n = 199) |
Europe (n = 175) |
P-value | |
|---|---|---|---|---|---|
Age, y (median, range) |
373 | 64 (19–85) |
64 (19–85) |
62 (19–83) |
0.03 |
| Gender (female) | 162 (43.3%) | 90 (45.2%) | 72 (41.1%) | 0.49 | |
| Histology | 374 | 0.003 | |||
| DLBCL | 266 (71.1%) | 127 (63.8%) | 139 (79.4%) | ||
| Transformed lymphoma | 99 (26.5%) | 65 (32.7%) | 34 (19.4%) | ||
| PMBCL | 9 (2.4%) | 7 (3.5%) | 2 (1.2%) | ||
| Response to previous therapy | 371 | 0.43 | |||
| Relapsed | 99 (26.7%) | 47 (23.9%) | 51 (29.5%) | ||
| Refractory | 151 (40.7%) | 82 (41.6%) | 69 (39.9%) | ||
| Primary refractory | 122 (32.9%) | 68 (34.5%) | 53 (30.6%) | ||
| Previous treatment (for LBCL) | |||||
| Auto-SCT | 372 | 98 (26.3%) | 37 (18.8%) | 61 (34.9%) | 0.0006 |
| Allo-SCT | 372 | 8 (2.2%) | 3 (1.5%) | 5 (2.9%) | 0.48 |
| Therapy lines (excl. H&B, median, IQR) | 372 | 3 (2–4) | 3 (2–5) | 3 (2–4) | <0.0001 |
| Holding therapy | 357 | 89 (24.9%) | 50 (27.3%) | 39 (22.4%) | 0.33 |
| Bridging therapy | 371 | 296 (79.8%) | 138 (70.4%) | 158 (90.3%) | <0.0001 |
| Therapy lines (incl. H&B, median, IQR) | 372 | 4 (3–5) | 4 (3–6) | 4 (3–5) | 0.02 |
| CAR T-cell product | 374 | <0.0001 | |||
| Axi-cel | 214 (57.2%) | 169 (84.9%) | 45 (25.7%) | ||
| Tisa-cel | 160 (42.8%) | 30 (15.1%) | 130 (74.2%) | ||
| Status at Indication for CAR-T therapy | |||||
| ECOG (median, IQR) | 374 | 1 (0–1) | 1 (0–1) | 1 (0–1) | 0.28 |
| Ann Arbor stage ≥ 3 | 374 | 81 (21.7%) | 38 (19.1%) | 43 (24.6%) | 0.21 |
| IPI (median, IQR) | 366 | 3 (2–4) | 3 (2–4) | 3 (2–3.5) | 0.07 |
| END | 374 | 265 (70.9%) | 158 (79.4%) | 107 (61.1%) | 0.002 |
| History of/active CNS disease | 314 | 34 (15.9%) | 14 (7.1%) | 20 (17.1%) | 0.008 |
LDH (U/L, median, range) |
353 | 295 (105–3487) | 271 (118–2879) | 321 (105–3478) | 0.02 |
| Inflammation markers at lymphodepletion | |||||
| CRP (mg/dL, median, range) | 371 | 1.1 (0.03–26.08) | 1.3 (0.04–26.08) | 1.1 (0.03–22.50) | 0.84 |
| Ferritin (ng/mL, median, range) | 332 | 529.5 (3–12,843) | 425 (8-12,843) | 675 (3–6896) | 0.004 |
- Patients’ characteristics. Statistical significance (P < 0.05) between US and European patients was determined by Fisher exact test for incidence rates and Mann-Whitney test for continuous variables. The center-specific upper limit of normal for LDH was 214–378 U/L, for CRP was 0.5 mg/dL, and for Ferritin was 400 ng/mL. P values < 0.05 are highlighted in bold.
- Axi-cel = Axicabtagene ciloleucel; CAR = chimeric antigen receptor; CNS = central nervous system; CRP = C-reactive protein; DLBCL = diffuse large B-cell lymphoma; ECOG = Eastern Cooperative Oncology Group performance status; END = Extranodal disease; H&B = holding and bridging therapy; IPI = international prognostic index; IQR = interquartile range; LBCL = large B-cell lymphoma; LDH = lactate dehydrogenase; PMBCL = primary mediastinal B-cell lymphoma; SCT = stem cell transplantation; Tisa-cel = Tisagenlecleucel; US = United States.
When studying other baseline demographic and disease features by geographic region, we found a higher median age in the US patients (64 versus 62 y; P = 0.03) (Table 1). Transformed lymphoma was more commonly observed in the US cohort (33% versus 19%), while patients with active or history of central nervous system (CNS) disease were more frequent in the EU cohort (17% versus 7%; P = 0.008). The number of prior treatment lines excluding bridging/holding therapy was higher in the US patients (P < 0.001), whereas a higher proportion of EU patients had received a prior ASCT (35% versus 19%; P < 0.001). Axi-cel was more commonly applied in the US cohort (169/199 patients, 85%), whereas tisa-cel was more commonly applied in the EU (130/175 patients, 74%; Suppl. Table 3). Of note, tisa-cel-treated patients had prolonged vein-to-vein intervals and were enriched for high-risk baseline features (Suppl. Tables 4 and 5). Overall, these data indicate that EU patients displayed a prognostically adverse risk profile before CAR-T infusion, consisting of protracted CAR-T logistics, higher tumor volume (represented by higher LDH as a surrogate marker28.), and more pronounced systemic inflammation (represented by higher ferritin26.).
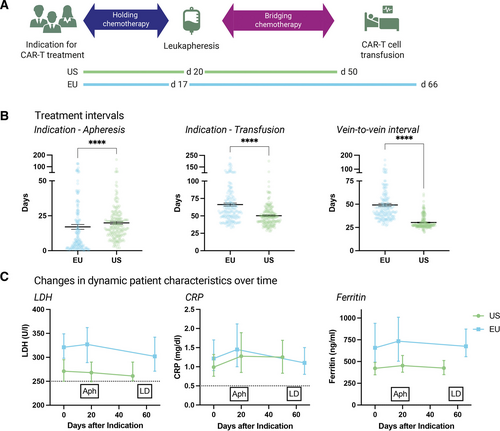
Logistics of CAR T-cell therapy: Vein-to-vein intervals are significantly longer for patients treated in Europe compared with US CAR T-cell patients, leading to significantly longer intervals between indication and CAR T-cell infusion in the EU. (A) Timelines depicting mean time intervals in days between indication, leukapheresis, and CAR T-cell infusion in European and US patients. Holding therapy denotes an antineoplastic treatment applied between indication and apheresis, whereas bridging therapy is used for a treatment applied after apheresis, but before CAR T-cell infusion. (B) Treatment intervals between indication to therapy, leukapheresis, and CAR T-cell infusion for EU and US patients. Box and whiskers describe the mean with standard error of mean (SEM). Significance was determined by Mann-Whitney test (****P < 0.0001). (C) Dynamic patient characteristics of tumor burden (LDH) and baseline inflammation (CRP, Ferritin) over time after indication for CAR T-cell treatment. Box and whiskers describe the median with 95% confidence intervals. Dotted lines mark upper limits of normal. Aph = leukapheresis; CAR = chimeric antigen receptor; CRP = C-reactive protein; EU = Europe; LD = lymphodepleting chemotherapy; LDH = lactate dehydrogenase; US = United States.
Patterns of holding and bridging therapy differ by geographic region
The majority of patients received an intermediary therapy between indication and CAR-T infusion (307/368, 83%; Suppl. Figure 1A). This included 89 of 357 patients (25%) receiving holding therapy, and 296 of 371 patients (80%) receiving bridging therapy. Across all patients, intermediary therapy included chemotherapy in 215 patients (57%), immunotherapy in 27 patients (7%), targeted small molecule (SM) therapy in 30 patients (8%), and radiotherapy in 65 patients (17%) (Suppl. Figure 1A). In total, 61 patients (16%) received neither holding nor bridging therapy. Bridging therapy was more frequently applied in patients receiving tisa-cel as opposed to axi-cel (94% versus 73%; P < 0.0001; Suppl. Figure 1B). An overall response rate of 27% was noted across all intermediary therapies. Of interest, the highest response rates were observed with immunotherapy-containing regimens (38%), followed by chemoimmunotherapy ([CIT], 35%) and SM therapies (28%) (Suppl. Figure 1C). Patients that either did not receive or responded to holding or bridging therapy were over-represented in the group that subsequently responded to CD19 CAR-T (Figure 2A). Serum LDH levels were significantly reduced (0.9-fold change; P < 0.001; Figure 2B) after therapy among patients responding to intermediary therapy. Conversely, nonresponse to intermediary therapy was associated with a significant increase in serum CRP (1.3-fold; P < 0.001) and ferritin levels (1.2-fold; P < 0.001) (Figure 2B).
When comparing the patterns of intermediary therapies by region, we found similar frequencies of holding therapy in the EU and US cohorts (22% versus 27%; P = 0.33). In contrast, we observed a higher frequency of bridging therapy in EU patients (90% versus 70%; P < 0.001) (Figure 2C). Consequently, a higher proportion of patients treated in the US did not receive any intermediary therapy (EU versus US: 6% versus 26%; P < 0.001). The applied holding and bridging strategies also differed, with more conventional chemotherapy (28% versus 13%; P = 0.006) and CIT (66% versus 34%; P < 0.001) in the EU compared with more frequent use of glucocorticosteroids (9% versus 25%; P < 0.001), radiotherapy and SMs (eg, BTK or PI3K inhibitors) in the US cohort (Suppl. Figure 1; Figure 2D–2E). The response rate to holding therapy was 41% in the EU (39 evaluable patients) compared with 8% in the US (24 evaluable patients) (Figure 2D; P = 0.005). The response rate to bridging therapy was also higher in the European patients (35% versus 19%; P = 0.006) (Figure 2E). Taken together, these data highlight differences in the application of intermediary therapies by geographic region.
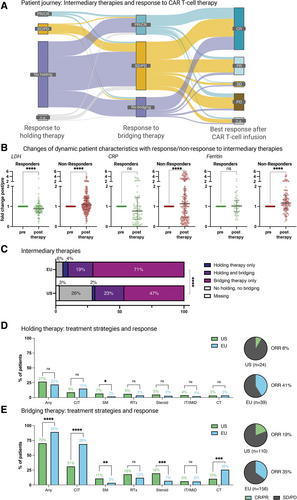
Significantly more patients receive intermediary therapies (applied between indication and CAR T-cell infusion) in European than in US treatment centers. (A) Sankey plot depicting the patients’ journey between indication for CAR T-cell treatment and infusion, illustrated by response to intermediary therapies and after CAR T-cell infusion. (B) Changes in normalized LDH, CRP, and Ferritin levels in responders and nonresponders to intermediary therapies. Pretreatment values were set as 1, and changes after therapy were calculated as fold change of the pretreatment value. Box and whiskers describe the median with interquartile range. Significance was determined by Wilcoxon test (****P < 0.0001). (C) Relative distribution of patients receiving holding and/or bridging therapies (n = 374), according to the region. Significance was determined by χ2 test (****P < 0.0001). (D) Left: Frequency of patients receiving holding therapy, and strategies used for holding. For patients receiving >1 treatment strategy, multiple allocations were possible. Significance was determined by Fisher exact test (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). Right: Objective response rate (CR + PR) of holding therapy in patients with evaluable disease assessment. (E) Left: Frequency of patients receiving bridging therapy, and strategies used for bridging. For patients receiving >1 treatment strategy, multiple allocations were possible. Significance was determined by Fisher exact test (*P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001). Right: Objective response rate (CR + PR) of bridging therapy in patients with evaluable disease assessment. CAR = chimeric antigen receptor; Chemo = chemotherapy (eg, ICE, when applied without eg, anti-CD20 antibody); CIT = chemoimmunotherapy (including rituximab+polatuzumab vedotine); CR = complete response; CRP = C-reactive protein; Immuno = immunotherapy (eg, rituximab monotherapy); LDH = lactate dehydrogenase; n.a. = not assessed; Ns = not significant; ORR = objective response rate; PD = progressive disease; PR = partial response; RTx = radiotherapy; SD = stable disease; SM = small molecule (eg, ibrutinib and copanlisib); US = United States.
Comparable product-specific toxicity and NRM rates by region
Severe CRS and tocilizumab application rates were comparable between patient cohorts (Figure 3A; Suppl. Table 6). However, an increased rate of grade ≥3 ICANS was noted in the US patient cohort (25% versus 9%; P < 0.001; Figure 3B), most likely due to the predominance of axi-cel (Suppl. Figure 2).15.,16. Concomitantly, high-dose glucocorticosteroids were more frequently applied in the US (48% versus 37%; P = 0.04) and axi-cel (52% versus 31%; P < 0.001) patient cohorts (Suppl. Table 6). With a 1-year NRM rate of 10.7% versus 4.8% in EU versus US patients, therapy-related mortality was not significantly different between patient cohorts (P = 0.31; Figure 3C). Furthermore, we did not observe significant differences in 1-year NRM by CAR product (tisa-cel versus axi-cel: 9.4% versus 6.0%, P = 0.91; Suppl. Figure 2). The duration of hospitalization was prolonged in the EU cohort (median 15 versus 13 in-patient days; P < 0.001; Figure 3D).
Inferior survival outcomes in European patients are driven by disease- and host-intrinsic differences and CAR-T product
The analysis of PFS and overall survival (OS) across study cohorts revealed significantly inferior survival outcomes in the patients treated in the EU (Figure 4A and 4B). Median PFS was 3.1 versus 9.2 months (P < 0.001), while median OS was 10.9 months versus not-reached (P < 0.001) for EU versus US treated patients, respectively. This translated into a significantly increased hazard ratio (HR) of 1.8 (95% confidence interval [CI], 1.4-2.3) and 1.9 (95% CI, 1.4-2.7) for PFS and OS, respectively, in EU patients. The best overall response rate was higher in the US cohort at 74% compared with 62% in the EU cohort, with a complete response rate of 58% versus 38% (Figure 4E). Notably, the survival differences were mirrored when directly comparing the CAR products, with inferior outcomes in the patients treated with tisa-cel (Suppl. Figure 3). These differences were also evident for PFS in both the EU and US subcohorts, and for OS in the European patients (Figure 4C and 4D).

Differences in CAR T-cell–associated toxicities are driven by the cell product. (A) Relative distribution of highest-grade CRS in EU and US patients. (B) Relative distribution of highest-grade ICANS in EU and US patients. (C) Cumulative incidence curves for nonrelapse mortality in European and US patients, calculated from CAR T-cell infusion. Significance was assessed by log-rank test. (D) Duration of postinfusion in-patient hospitalization for European and US patients in days. Box and whiskers describe the median with 95% confidence intervals. Significance was determined by Mann-Whitney test (****P < 0.0001). CAR = chimeric antigen receptor; CRS = cytokine-release syndrome; EU = Europe; ICANS = immune effector cell-associated neurotoxicity syndrome; Mo = months; NRM = nonrelapse mortality; US = United States.
To understand which specific variables, apart from differences in the use of CAR-T products, drove the survival differences observed in our study cohort, we performed univariate and multivariate modeling (Table 2). On univariate Cox regression analysis, we identified nonresponse to bridging, elevated serum ferritin and CRP levels, and tisa-cel use as particularly negative prognostic factors (all P < 0.001). Other adverse risk features included, among others, poor ECOG performance status and increased serum LDH (for detailed results see Table 2). The identified potential prognostic markers with a P ≤ 0.1 on univariate Cox regression were subsequently introduced into a multivariate Lasso model. CAR product use was excluded as we aimed to identify product-independent risk factors. Notably, only nonresponse to bridging (Lasso coefficient 0.35), serum ferritin at lymphodepletion (Lasso coefficient 0.1), and serum CRP at lymphodepletion (Lasso coefficient 0.01) were retained as independent risk factors of progression.
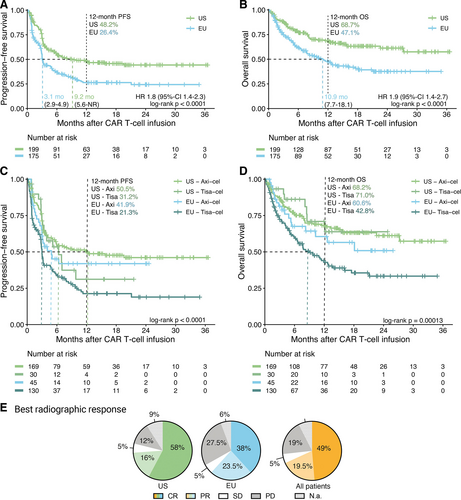
Progression-free survival, overall survival, and best radiographic response are significantly inferior for European compared with US patients after CAR T-cell therapy. (A–D) Kaplan-Meier estimates of progression-free (A and C) and overall (B and D) survival, calculated from the day of CAR T-cell infusion, for European and US patients, and stratified by CAR T-cell product used (C and D). Median survival (when reached) is reported with 95% confidence interval in the respective insets. Significance was assessed by log-rank test. (E) Pie charts depicting the best radiographic response after CAR T-cell infusion for European, US and all patients. CR percentages are reported in bold, and PR percentages in light colors. CAR = chimeric antigen receptor; CR = complete response; EU = Europe; Mo = months; N.a. = not assessed; NR = not reached; PD = progressive disease; PR = partial response; SD = stable disease; US = United States.
Axi-cel is associated with superior survival outcomes in low-risk patients
To further delineate which patients are particularly impacted by CAR product choice, we calculated a risk classification score based on the coefficients derived from our multivariate Lasso model (Table 2; n = 277). The continuous HR balancer was calculated as follows:
| Variable | n | Univariate HR (95% CI) |
P-value | High-risk Balancer Coefficients (Lasso Model) |
Confirmatory Lasso Model Coefficients |
|---|---|---|---|---|---|
| Response to bridging (SD/PD vs CR/PR or no bridging) | 346 | 2.1 (1.6-2.8) | <0.0001 | 0.35 (nonresponse) |
-a |
| Ferritin (fold change ULN, log2) | 332 | 1.2 (1.1-1.4) | <0.0001 | 0.10 | -a |
| CAR-T product (Tisa-cel vs Axi-cel) | 374 | 1.8 (1.4-2.3) | <0.0001 | -a | 0.04 (Tisa-cel) |
| CRP (fold change ULN, log2) | 371 | 1.1 (1.1-1.2) | <0.0001 | 0.01 | -a |
| ECOG (0-1 vs 2-4) | 374 | 1.9 (1.3-2.6) | 0.0002 | - | - |
Interval Apheresis-CAR-T (mo, log2) |
374 | 1.5 (1.2-1.9) | 0.001 | - | - |
| Presence of END | 374 | 1.6 (1.1-2.1) | 0.005 | - | - |
Interval Indication-CAR-T (mo, log2) |
374 | 1.4 (1.1-1.8) | 0.006 | - | - |
| LDH (fold change ULN, log2) | 353 | 1.3 (1.1-1.5) | 0.006 | - | - |
| Prior therapy lines (incl. H&B, log2) | 372 | 1.3 (1-1.7) | 0.020 | - | - |
| Ann Arbor Stage (III/IV vs I/II) | 374 | 1.5 (1.1-2.1) | 0.020 | - | - |
| Response to previous therapy (refractory vs relapsed) | 371 | 1.4 (1.0-1.9) | 0.039 | - | - |
Diagnosis (LBCL vs transformed LBCL) |
374 | 1.4 (0.99-1.9) | 0.060 | - | - |
| Bulky disease | 335 | 1.4 (0.96-2.1) | 0.082 | - | - |
| HR balancer | 277 | 1.11 | |||
| History of/active CNS disease | 314 | 1.4 (0.92-2.2) | 0.12 | ||
| Interval initial diagnosis-indication (years, log2) | 175 | 0.96 (0.89-1) | 0.25 | ||
| History of auto-SCT | 372 | 0.85 (0.63-1.2) | 0.29 | ||
| Age (years) | 373 | 1 (0.98-1) | 0.36 | ||
| Interval Initial diagnosis – first relapse (years, log 2) | 175 | 0.94 (0.83-1.1) | 0.36 | ||
| History of allo-SCT | 372 | 1.2 (0.5-2.9) | 0.68 | ||
| Prior therapy lines (excl. H&B, log2) | 372 | 1 (0.92-1.1) | 0.93 | ||
Interval Indication – apheresis (mo, log2) |
361 | 1 (0.85-1.3) | 0.77 |
- Factors associated with PFS in univariate and multivariate analysis. For multivariate analysis, only variables with P ≤ 0.1 in univariate Cox regression analysis were included. CAR T-cell product, however, was omitted for the definition of the multivariate model to identify product-independent variables with significant influence on PFS. A Lasso penalized regression model was used. Missing ferritin values at the time of lympho depletion were replaced with values from apheresis or indication (n = 12) for multivariate analysis. A confirmatory Lasso model was calculated using all variables identified in univariate analysis with the exception of those already included in the HR balancer (response to bridging, ferritin, and CRP), but now including the used CAR T-cell product to allow an estimate of the influence of product choice. P values <0.05 in univariate analysis and Lasso coefficients with relevance for the multivariate model are highlighted in bold.
- a Excluded from Lasso analysis.
- CAR = chimeric antigen receptor; CI = confidence interval; CNS = central nervous system; CR = complete response; CRP = C-reactive protein; ECOG = Eastern Cooperative Oncology Group performance status; END = extranodal disease; H&B = holding and bridging therapy; HR = hazard ratio; LDH = lactate dehydrogenase; mo = months; PD = progressive disease; PFS = progression-free survival; PR = partial response; SCT = stem cell transplantation; SD = stable disease; Tisa-cel = tisagenlecleucel; ULN = upper limit of normal.
HR balancer = 0.35 × 1 (bridging nonresponse) + 0.1 × ferritin (fold change ULN, log2) + 0.01 × CRP (fold change ULN, log2)
A high HR balancer indicates an increased risk of poor post-CAR-T survival outcomes. Importantly, we found that the patients treated in the EU and with tisa-cel displayed significantly increased scores (Figure 5A), highlighting cohort-level differences in key prognostic markers. Furthermore, patients could be risk-stratified into a low- versus high-risk profile (discriminatory threshold: HR balancer of 0.52), which resulted in a clear separation of PFS and OS survival curves (Suppl. Figure 4). For example, comparing high- versus low-risk patients, the median PFS was 2.9 months versus 11.3 months (log-rank P < 0.0001), and the median OS was 5.9 months versus not-reached (log-rank P < 0.001), respectively.
Next, we studied survival outcomes by CAR product and risk profile. Interestingly, we did not find a significant PFS difference in the patients with a particularly high-risk profile (P = 0.49) (Figure 5B). However, tisa-cel-treated patients with a low-risk profile exhibited markedly inferior PFS (HR = 1.9 [95% CI, 1.2-2.9]). In this subgroup, median PFS for tisa-cel was 7.4 months versus not-reached for axi-cel. The described observations were consistent when studying OS (Suppl. Figure 5). Additionally, in a confirmatory Lasso model incorporating the HR balancer, tisa-cel use represented the only HR balancer-independent variable also associated with PFS (Table 2). A trend toward increased NRM was observed in patients with high-risk disease (13 versus 6%; P = 0.059; Suppl. Figure 6). Importantly, both a multivariable PRE balancer model that only incorporated variables assessed at CAR-T indication (eg, ferritin, LDH, CRP, and extranodal disease) and a model containing relevant interactions (including CAR-T product × response to bridging) largely confirmed the HR balancer-based observations (Suppl. Tables 7 and 8). Indeed, HR and PRE balancers were strongly correlated (Spearman r = 0.8; P < 0.001; Suppl. Figure 7). Taken together, these data suggest a sweet spot for product-related efficacy differences, with axicabtagene-ciloleucel being particularly efficacious in low-risk patients.
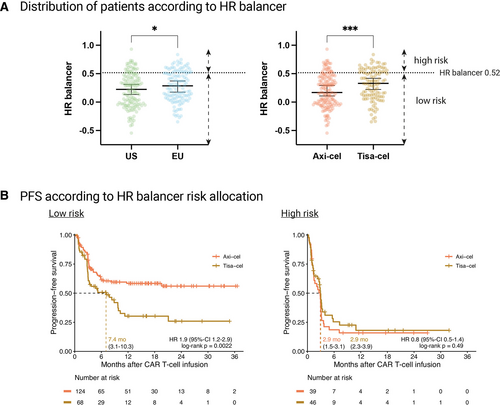
Patients from US and Europe, and also axi-cel vs tisa-cel-treated patients, differ in their pre-CAR risk profile, as assessed by HR balancer. Progression-free survival for low-risk patients receiving axi-cel is superior in comparison to low-risk patients receiving tisa-cel, whereas the choice of CAR T-cell product does not influence outcomes in high-risk patients. (A) Left: Distribution of European and US patients, according to their HR balancer value. Right: Distribution of patients receiving axi-cel or tisa-cel, according to their HR balancer value. The cutoff between low- and high-risk patients was chosen for maximum difference in progression-free survival for both groups. Box and whiskers describe the median with 95% confidence intervals. Significance was determined by Mann-Whitney test (*P < 0.05; ***P < 0.001). (B) Kaplan-Meier estimates of progression-free survival in patients receiving axi-cel or tisa-cel for HR balancer low-risk (left) and high-risk (right) patients, calculated from the day of CAR T-cell infusion. Median survival (when reached) is reported with 95% confidence interval in the respective insets. Significance was assessed by log-rank test. Axi-cel = axicabtagene-ciloleucel; CAR = chimeric antigen receptor; EU = Europe; HR = hazard ratio; Tisa-cel = tisagenlecleucel; US = United States.
DISCUSSION
In this multicenter real-world cohort of 374 patients receiving CD19 CAR-T for R/R LBCL, we demonstrate that patients treated in the EU cohort presented to therapy with higher pretreatment levels of systemic inflammation and tumor burden, longer vein-to-vein intervals, and more frequently received bridging therapy. Together with the frequent application of tisa-cel, which was associated with poor clinical outcomes in low-risk patients, these differences translated into inferior survival in the EU cohort. Based on the multivariate Lasso models, we allocated patient-individual risk balancers, which were significantly increased in EU and tisa-cel-treated patients.
The combination of adverse prognostic markers (eg, high LDH, and ferritin) and longer vein-to-vein intervals provides compelling evidence as to why more patients had to receive bridging, or did not only receive steroids as intermediary monotherapy in the EU cohort. It is notable that these differences were present before CAR-T indication, implying differences in referral patterns or patient selection. Importantly, an inflammatory cytokine environment in the peripheral blood has been linked to an immunohostile microenvironment with upregulated interferon signaling and immune checkpoint expression on the tumor, as well as high levels of myeloid-derived suppressor cells.26. This in turn negatively influences CAR T-cell expansion and confers resistance of the underlying lymphoma to CD19 CAR-T.25. Similarly, a more frequent use of intermediary therapies may introduce additional genomic events, which may lead to decreased CAR-T efficacy because more complex lymphomas are more likely to be CAR-T resistant.29.,30.
In this report, we provide a detailed description of intermediary therapies (holding and bridging) that patients receive between the time they are indicated for CAR-T cell therapy and the time they are infused. We expand the current description of these intermediary therapies to include holding therapy, in which we uncovered that a quarter of patients required treatment between the time CAR-T cell therapy was indicated and the time of leukapheresis. This was in addition to the 80% of patients that required bridging therapy after leukapheresis while awaiting CAR-T cell manufacturing, and consistent with prior real-world reports.9.,11.,31. Overall, intermediary therapies are often necessary due to delays in insurance or treatment approval, long manufacturing times, and/or high-risk disease features. It is unclear whether the requirement for intermediary therapies directly worsen CAR-T cell outcomes or if they instead reflect higher risk tumor biology. In general, nonresponse to systemic therapies not only during bridging,22. but also for frontline therapy,32. defines a group at high risk for CAR-T cell resistance.33. Moreover, holding therapies may affect the quality of T cells obtained from leukapheresis. Patients not receiving bridging/holding or receiving steroids alone (20%), more frequently present in the US cohort, exhibited markedly superior survival compared with patients that were treated with more intensive intermediary therapies (Suppl. Figure 8). The higher use of bridging therapy in the EU cohort is likely related to adverse baseline risk features that might also worsen during the longer indication-to-infusion interval. Indeed, we noted a significant interaction between tisa-cel use and the vein-to-vein interval (Suppl. Table 8), potentially because tisa-cel production initially required negative results of microbiological testing of the apheresis product, leading to prolonged manufacturing intervals. The observation that many patients exhibit worsening markers of tumor burden and inflammation in the period awaiting CAR-T infusion underlines that both the prompt availability of leukapheresis slots and short manufacturing intervals represent key constituents of product efficacy. Better intermediary therapies are needed to facilitate tumor debulking, modulate the tumor microenvironment, and/or reduce systemic inflammation (Figure 2B). Ideally, their influence on outcomes after CD19 CAR-T therapy will be evaluated prospectively.
In the absence of comparative randomized controlled trials, real-world reports remain the most relevant data source to delineate the efficacy of different CAR products. To date, findings have been mixed with some trials demonstrating equipoise,11.,12.,34. while others reported superiority of axi-cel even after multivariate adjustment.9.,12.,35. Our study builds on this body of evidence, and underlines that the advantage of axi-cel may be restricted to patients with a low-risk profile. Conversely, patients with an elevated HR balancer performed poorly with both CAR products (median PFS 2.9 months). These data suggest a preference for axi-cel in low-risk patients and provide a rationale for moving axi-cel into earlier therapy lines, as was recently successfully demonstrated in the ZUMA-72. and ZUMA-1236. trials. In line with prior reports, axi-cel was associated with increased toxicity in our study.9.,11.,37. Surprisingly, NRM was still numerically higher with tisa-cel, which stands in contrast to previous work,9.,11. although this likely reflects the higher disease burden and risk profile at baseline observed in the tisa-cel cohort (Suppl. Tables 3 and 7). The fact that tisa-cel was enriched in the high-risk group may reflect physician preference due to the expected toxicity profiles. However, our survival data highlight that any potential gains in decreased toxicity need to be carefully weighed with a potential significant loss of efficacy. This caveat appears particularly relevant in patients that may be older or frail, but do not present with other high-risk features (eg, low CRP and ferritin).
This study has several relevant limitations. It represents a nonrandomized retrospective analysis and only includes patients that actually received their CAR product (no screening or manufacturing failures). Patients receiving lisocabtagene maraleucel were not included. Furthermore, the US patient cohort was derived from a single center (Moffitt Cancer Center), although the outlined toxicity and efficacy results of this center largely reflect US real-world results within the Center for International Blood and Marrow Transplant Research registry.6.,38. As patients from only 2 European countries (Germany and Spain) were included, outcomes from these patients are not necessarily representative for all EU-treated CAR-T patients. Although we studied efficacy in the context of patient-individual risk profiles (HR balancer/PRE balancer), we did not perform propensity-score or inverse probability weighting matching analysis.34. Still, we see several important clinical implications and consequences from this report. The described nomenclature of intermediary therapies (holding versus bridging therapy) provides a blueprint for reporting in trial protocols. Most importantly, we demonstrate that the observed disparities in EU and US outcomes following CD19 CAR-T are associated with higher-risk disease and logistic features, as well as tisa-cel use, in EU patients. Next-generation clinical trials may use bridging response as a criterion to select high-risk patients to test novel sequential or combinatorial strategies. Finally, further efforts are urgently needed to provide solutions to the logistic challenges that may limit the successful application of CAR-T therapy (eg, bridging, manufacturing, vein-to-vein intervals)—particularly as CAR-T moves into further indications, disease entities, and geographic regions.3.,39.-43.
In conclusion, these findings reveal important discrepancies in CAR T-cell delivery and patient characteristics between the EU and US patient cohorts, resulting in notable differences in clinical outcomes. Additionally, we demonstrate that axi-cel is particularly efficacious in a low-risk group of patients. Future studies will need to consider subtleties in patient characteristics and logistics to optimize patient selection and CAR-T treatment outcomes.
ACKNOWLEDGMENTS
First and foremost, we would like to thank all patients who contributed to the results in this study. We would like to thank our generous funding sources. Results of these analyses were presented at the American Society of Hematology (ASH) Annual Meeting 2022 in New Orleans.
AUTHOR CONTRIBUTIONS
VB, AP, KR, MS, and MJ did conceptualization. VB, AP, KR, GI, VJ, UH, OP, SK, VBl, JA, LF, GJ, KP, BA, RM, AW, CS, OA, PG, EH, LB, AM, FL, MvB, PB, MS, and MJ did investigation. VB, AP, and KR performed formal analysis and visualization. VB, KR, VJ, and EH did methodology. VJ and EH gave statistical advice. VB, KR, MS, and MJ performed writing original draft. VB, AP, KR, GI, VJ, UH, OP, SK, VBl, JA, LF, GJ, KP, BA, RM, AW, CS, OA, PG, EH, LB, AM, FL, MvB, PB, MS, and MJ performed writing, review, and editing. All authors read and approved the final article.
DISCLOSURES
VB: Amgen: Honoraria; Celgene: Research Funding; Pfizer: Honoraria; Kite/Gilead: Research Funding, Honoraria; Novartis: Honoraria. KR: Kite/Gilead: Research Funding and travel support. Novartis: Honoraria. GI: Consultancy and Honoraria: Novartis, Roche, Kite/Gilead, Bristol-Myers Squibb, Abbvie, Janssen, Sandoz, Miltenyi. UH: Consultancy and Honoraria: Amgen, BMS/Celgene, CSL Behring, GSK, Janssen, Kite/Gilead, Novartis, Sanofi. OP: Honoraria or travel support: Gilead, Jazz, MSD, Novartis, Pfizer and Therakos. Research support: Incyte and Priothera. Consultancy: Equillium Bio, Jazz, Gilead, Novartis, MSD, Omeros, Priothera, Shionogi and SOBI. SK: Celgene/Bristol-Myers Squib: Honoraria. V Blumenberg: Novartis: Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Celgene: Research Funding; Janssen: Research Funding. PG: Travel support: Gilead. LB: Honoraria: Novartis, Celgene/BMS, Astellas, Gilead, Abbvie, Jazz Pharmaceuticals, Pfizer, Janssen; Consultancy: Novartis, Celgene/BMS, Gilead, Abbvie, Jazz Pharmaceuticals, Pfizer, Janssen; Research Funding: Jazz Pharmaceuticals, Bayer Oncology. AM: Honoraria: Novartis, Kite/Gilead, Celgene/BMS, Miltenyi Biomedicine. FL: has a scientific advisory role with Kite, a Gilead Company, Novartis, Celgene/Bristol-Myers Squibb, GammaDelta Therapeutics, Wugen, Amgen, Calibr, and Allogene; is a consultant with grant options for Cellular Biomedicine Group, Inc.; and receives research support from Kite, a Gilead Company, Novartis, and Allogene; and reports that his institution holds unlicensed patents in his name in the field of cellular immunotherapy. MvB: Consultancy, Research Funding and Honoraria: MSD Sharp & Dohme, Novartis, Roche, Kite/Gilead, Bristol-Myers Squibb, Astellas, Mologen, and Miltenyi. PB: declares having received honoraria from Amgen, BMS, Gilead, Incyte, Miltenyi Biotec, Novartis and Pfizer not related with the present article. MS: Morphosys: Research Funding; Novartis: Consultancy, Research Funding; Janssen: Consultancy; Seattle Genetics: Research Funding; AMGEN: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Kite/Gilead: Consultancy, Honoraria, Research Funding; Roche AG: Consultancy, Research Funding. MDJ: Kite/Gilead: Consultancy/Advisory, Novartis: Consultancy/Advisory, BMS: Consultancy/Advisory, Takeda: Consultancy/Advisory. All the other authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
This work was supported by a Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) research grant provided within the Sonderforschungbereich SFB-TRR 388/1 2021 – 452881907, and DFG research grant 451580403 (to MS). The work was further supported by the Bavarian Elite Graduate Training Network (to MS), the Wilhelm-Sander Stiftung (to MS, project no. 2018.087.1), the Else-Kröner-Fresenius Stiftung (to MS), the Bavarian Center for Cancer Research (BZKF), and NCI Cancer Center Support Grant P30 CA076292. VLB, KR, and VB were funded by the Else-Kröner Forschungskolleg (EKFK) within the Munich Clinician Scientist Program (MCSP).