Haploidentical Versus Matched Sibling Donor Hematopoietic Stem Cell Transplantation for Adult Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia: A Study From the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation
Data presented in part at the ASH 2021 Hybrid Congress (publication number 1841).
The scientific boards of the ALWP of the EBMT approved this study.
AN, ML and MM have full access to all study data (available upon data-specific request).
Supplemental digital content is available for this article.
Abstract
The results of haploidentical stem cell transplantation (haploHCT) for patients with acute lymphoblastic leukemia (ALL) transplanted in active disease remain largely unknown. We retrospectively analyzed adult patients with R/R ALL who underwent haploHCT or matched sibling donor (MSD-HCT) as a first transplantation between 2012 and 2020. The analysis comprised 274 patients, 94 had a haploHCT, and 180 had an MSD-HCT. The median follow-up was 32 months. The median age was 33 (range 18–76) and 37 (18–76) years in the haplo- and MSD-HCT groups, respectively. Post-transplant cyclophosphamide (PTCy) was used in 88% of haploHCT and in 4% of the MSD-HCT group. Graft-versus-host disease grade III–IV was higher in haploHCT than in the MSD-HCT group (18% versus 9%; P = 0.042). The 2-year chronic (c) graft-versus-host disease rates were 17% versus 33% (hazard ratio [HR] = 0.56; P = 0.14), respectively. By multivariate analysis, relapse incidence, and leukemia-free survival were not significatively different between the transplant groups, while nonrelapse mortality (NRM) was significantly higher (25% versus 18% at 2 years; HR = 2.03; P = 0.042) and overall survival (OS) lower (22% versus 38% at 2 years; HR = 1.72; P = 0.009) in the haploHCT group compared with the MSD-HCT group. We conclude that the 2-year OS of R/R ALL patients undergoing MSD transplants is significantly better than in haploHCT with a higher NRM in the latter.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (alloHCT) is a standard of care for adult patients with acute lymphoblastic leukemia (ALL) in first or subsequent complete remission (CR).1, 2 Results of alloHCT for ALL in CR1 improved over time in relation to nonrelapse mortality (NRM), relapse incidence (RI), and overall survival (OS).3 The outcomes for patients with primary refractory and relapsed disease are significantly inferior; however, in this population, alloHCT may be the only curative option with up to a 20% chance of long-lasting remissions.4-6
Human leukocyte antigen (HLA) matched sibling donors (MSD) are considered optimal; however, their availability is restricted to about 25%–30% of individuals. A chance to identify a HLA-matcheded unrelated donor (MUD) varies with racial and ethnic background and ranges from 16% to 75%.7 For patients lacking HLA-matched donors, transplantations from haploidentical ones (haploHCT), available for a vast majority of patients, have become a widely accepted alternative.8 Several studies showed similar efficacy of haploHCT in comparison with MSD-HCT or MUD-HCT for ALL patients in CR.9-12 Moreover, according to results of a prospective study from China including patients in CR with detectable measurable residual disease (MRD), haploHCT was associated with reduced RI as well as improved leukemia-free survival (LFS) and OS compared to MSD-HCT.13 The authors postulated a stronger graft-versus-leukemia (GVL) effect after haploHCT, related to HLA disparity. In that study antigraft-versus-host disease (GVHD) prophylaxis was based on the use of high doses of antithymocyte globulin (ATG).13
Nowadays, the use of post-transplantation cyclophosphamide (PTCy) has become the most common strategy for GVHD prophylaxis in non-T-cell depleted haploHCT.14-17 In a recent registry-based study comparing PTCy to ATG as a backbone of immunosuppression for ALL patients undergoing unmanipulated haploHCT in CR, PTCy has been shown superior to ATG with reduced RI and improved LFS and OS while no effect on the incidence of either acute(a)or chronic(c) GVHD.18 So far, studies comparing MSD-HCT and haploHCT in a setting of ALL were focused on patients in CR.9-13 However, it may be assumed that for transplantations performed in active disease, the role of potential GVL reaction associated with HLA disparity is particularly important. Therefore, the aim of this study was to compare outcomes of non-T-cell depleted haploHCT and MSD-HCT in patients with relapsed/refractory (R/R) ALL.
SUBJECTS AND METHODS
Study design and data collection
This was a retrospective, multicenter analysis based on the registry of the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT). The EBMT is a nonprofit, scientific society representing more than 600 transplant centers, mainly located in Europe, which are required to report all consecutive stem cell transplantations and follow-ups once a year. Data are entered, managed, and maintained in a central database. EBMT centers commit to obtaining informed consent according to the local regulations applicable at the time of transplantation and report pseudonymized data to the EBMT. The validation and quality control program includes verification of the computer print-out of the entered data, cross-checking with the national registries, and on-site visits to selected teams. The study was approved by the ALWP of the EBMT and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Criteria for selection
The study included adult patients aged ≥18 years old with primary R/R ALL who underwent their first alloHCT between 2012 and 2020 from either non-T-cell depleted haploidentical donor or MSD with peripheral blood or bone marrow as a source of stem cells and following myeloablative (MAC) or reduced-intensity conditioning (RIC). The use of cord blood as a source of stem cells as well as transplantations with ex vivo T-cell depletion were exclusion criteria.
Statistical analysis
LFS was the primary study endpoint. Secondary endpoints were the incidence of engraftment, OS, RI, NRM, the incidence of aGVHD and cGVHD, and GVHD- and relapse-free survival (GRFS).19 All patients who met the inclusion criteria were divided into 2 groups according to donor type. Patients who underwent transplantation from a haploidentical donor were compared with those transplanted from an MSD. Patients’ characteristics were compared using the Mann-Whitney test for continuous variables, and the chi-squared or Fisher's exact test for categorical variables.20 The probabilities of OS, LFS, and GRFS were calculated using the Kaplan-Meier method. The probabilities of RI, NRM, and acute and cGVHD were estimated using cumulative incidence curves.21 Univariate comparisons were performed with the log-rank test for LFS, OS, and GRFS, and Gray's test was used to compare cumulative incidence functions.22 Multivariate analysis was performed using a Cox proportional-hazards model which included variables differing significantly between the groups, factors known to be associated with outcomes, plus a center frailty effect to take account of the heterogeneity across centers. Results were expressed as the hazard ratio (HR) with a 95% confidence interval (95% CI). All tests were 2-sided with a type 1 error rate fixed at 0.05. Statistical analyses were performed with SPSS 27.0 (IBM Corp., Armonk, NY, USA) and R 4.1.1 (R Core Team [2021]; R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria, URL https://www.R-project.org/).
RESULTS
Patients and transplantation procedure
Out of 274 patients who met inclusion criteria, 94 (34.3%) patients were treated with haploHCT and 180 (65.7%) patients underwent MSD-HCT. Median age was 32.7 (range 18.1–76.1) and 37.4 (18.5–75.6) years in haploHCT and MSD-HCT, respectively (P = 0.24). Baseline patients’ characteristics, such as sex, Karnofsky performance status score (KPS), and subtype of ALL (Philadelphia [Ph] positive B-/Ph negative B-/T-ALL) did not differ between the studied groups. The percentage of patients with primary refractory disease was lower in haploHCT than in the MSD-HCT group (31.9% versus 55.0%), while more haploHCT patients were in second or subsequent relapse (33% versus 11.1%, P < 0.0001). The patient characteristics are shown in Table 1. Fewer patients undergoing haploHCT compared with MSD-HCT received MAC (67% versus 83.9%, P = 0.001) and regimens based on total body irradiation (TBI) (31.9% versus 68.3%, P < 0.0001). PTCy was used in 88.3% of the haploHCT, while only in 3.9% of the MSD-HCT, respectively. The use of ATG was similar in both groups. The most frequent drugs used as a GVHD prophylaxis were cyclosporine (CsA) combined with mycophenolate mofetil (MMF) in the haploHCT group while CsA combined with methotrexate (MTX) in the MSD-HCT setting. The detailed transplant characteristics are shown in Table 2.
| HaploHCT (n = 94) | MSD-HCT (n = 180) | P | |
|---|---|---|---|
| Median age, y (min–max) | 32.7 (18.1–76.1) | 37.4 (18.5–75.6) | 0.24 |
| Patient sex | |||
| Male | 58 (62.4%) | 119 (66.5%) | 0.5 |
| Female | 35 (37.6%) | 60 (33.5%) | |
| Missing | 1 | 1 | |
| Diagnosis | |||
| Ph (–) B-ALL | 21 (22.3%) | 49 (27.2%) | 0.52 |
| Ph (+) B-ALL | 23 (24.5%) | 35 (19.4%) | |
| T-ALL | 50 (53.2%) | 96 (53.3%) | |
| Disease status at HCT | |||
| Refractory | 30 (31.9%) | 99 (55%) | <0.0001 |
| Relapse 1 | 33 (35.1%) | 61 (33.9%) | |
| Relapse ≥2 | 31 (33%) | 20 (11.1%) | |
| KPS | |||
| <90 | 35 (40.2%) | 61 (35.3%) | 0.43 |
| ≥90 | 52 (59.8%) | 112 (64.7%) | |
| Missing | 7 | 7 | |
| Patient CMV serological status | |||
| Negative | 15 (16.1%) | 46 (26.6%) | 0.053 |
| Positive | 78 (83.9%) | 127 (73.4%) | |
| Missing | 1 | 7 | |
| HCT-CI | |||
| 0 | 38 (66.7%) | 73 (57.5%) | 0.45 |
| 1 or 2 | 9 (15.8%) | 29 (22.8%) | |
| ≥3 | 10 (17.5%) | 25 (19.7%) | |
| Missing | 37 | 53 |
- (–) = negative; (+) = positive; B-ALL = B-acute lymphoblastic leukemia; CMV = cytomegalovirus; Haplo = haploidentical; HCT = hematopoietic cell transplantation; HCT-CI = hematopoietic cell transplantation-specific comorbidity index; KPS = Karnofsky performance status; MSD = matched sibling donor; Ph = Philadelphia; T-ALL = T-acute lymphoblastic leukemia.
| HaploHCT (n = 94) | MSD-HCT (n = 180) | P | |
|---|---|---|---|
| Median year of transplantation (min–max) | 2015.5 (2012–2020) | 2016 (2012–2020) | 0.65 |
| Donor/patient sex | |||
| no F->M | 68 (73.1%) | 125 (69.8%) | 0.57 |
| F->M | 25 (26.9%) | 54 (30.2%) | |
| Missing | 1 | 1 | |
| Type of conditioning | |||
| MAC | 63 (67%) | 151 (83.9%) | 0.001 |
| RIC | 31 (33%) | 29 (16.1%) | |
| Conditioning regimen | |||
| BuCy ± other | 3 (3.2%) | 18 (10%) | <0.0001 |
| BuFlu ± other | 52 (55.3%) | 25 (13.9%) | |
| FluMel ± other | 4 (4.3%) | 6 (3.3%) | |
| TBI | 30 (31.9%) | 123 (68.3%) | |
| Other | 5 (5.3%) | 8 (4.4%) | |
| Cell source | |||
| BM | 41 (43.6%) | 17 (9.4%) | <0.0001 |
| PB | 53 (56.4%) | 163 (90.6%) | |
| GVHD prevention | |||
| CsA | 1 (1.1%) | 14 (7.8%) | |
| CsA + MTX | 3 (3.2%) | 126 (70.4%) | |
| MTX + Tacro | 0 (0%) | 2 (1.1%) | |
| CsA + MMF | 53 (56.4%) | 21 (11.7%) | |
| CsA + Tacro | 0 (0%) | 2 (1.1%) | |
| CsA + MTX + MMF | 5 (5.3%) | 2 (1.1%) | |
| MMF + Tacro | 20 (21.3%) | 3 (1.7%) | |
| MMF + Siro | 7 (7.4%) | 0 (0%) | |
| Tacro + Siro | 2 (2.1%) | 2 (1.1%) | |
| Other | 3 (3.2%) | 7 (3.9%) | |
| missing | 0 | 1 | |
| PTCy | |||
| no | 11 (11.7%) | 1732 (96.1%) | <0.0001 |
| yes | 83 (88.3%) | 7 (3.9%) | |
| In vivo T-cell depletion | |||
| no in vivo TCD | 71 (75.5%) | 138 (77.1%) | 0.77 |
| n vivo TCD | 23 (24.5%) | 41 (22.9%) | |
| missing | 0 | 1 |
- BM = bone marrow; Bu = busulfan; CsA = cyclosporine; Cy = cyclophosphamide; F = female; Flu = fludarabine; Haplo = haploidentical; HCT = hematopoietic cell transplantation; M = male; MAC = myeloablative conditioning; Mel = melphalan; MMF = mycophenolate mofetil; MSD = matched sibling donor; MTX = methotrexate; PB = peripheral blood; PTCy = post-transplant cyclophosphamide; RIC = reduced-intensity conditioning; Siro = sirolimus; Tacro = tacrolimus; TBI = total body irradiation; TCD = T-cell depletion.
Engraftment, response, and GVHD
Engraftment rate was lower in the haploHCT compared to the MSD-HCT group (86.5% versus 96%, respectively, P = 0.005). CR rate after transplantation was 64.4% in the haploHCT group and 69.3% in the MSD-HCT group (P = 0.3). By univariate analysis, the incidence of aGVHD grade II–IV at day 180 did not differ between haploHCT and MSD-HCT (27.9% versus 21.4%, respectively, P = 0.25), while aGVHD grade III–IV was higher in haploHCT than in MSD-HCT group (17.8% versus 9%, respectively, P = 0.042). Conversely, the 2-year cGVHD and extensive cGVHD rates were 17.2% versus 32.5% (P = 0.012) and 5% versus 16.9% (P = 0.011), respectively.
Survival
The median follow-up was 32.1 months. One hundred sixty-eight and 100 of the R/R ALL patients that underwent haploHCT and MSD-HCT study patients died, respectively. The main causes of death after haploHCT and MSD-HCT were leukemia (63.9% versus 62.6%, respectively), infection (15.7% versus 18.2%), and GVHD (12.7% versus 11.1%) (Suppl. Table S1). At 2-year, RI was 56.6% versus 51.5% (P = 0.53), NRM was 25.4% versus 17.7% (P = 0.13); LFS was 18% versus 30.8% (P = 0.023); OS 21.6% versus 37.9% (P = 0.001) and GRFS 15.7% versus 19.4% (P = 0.06) in the haploHCT group compared with the MSD-HCT group, respectively.The transplant outcomes are shown in Table 3 and Figure 1.
| HaploHCT (n = 94) | MSD-HCT (n = 180) | P | |
|---|---|---|---|
| RI | 56.6% [45–66.6] | 51.5% [43.2–59.2] | 0.53 |
| NRM | 25.4% [16.7–35.1] | 17.7% [12.2–23.9] | 0.13 |
| LFS | 18% [10.4–27.2] | 30.8% [23.5–38.4] | 0.023 |
| OS | 21.6% [13.2–31.4] | 37.9% [29.8–46] | 0.001 |
| GRFS | 15.7% [8.7–24.6] | 19.4% [13.3–26.4] | 0.06 |
| aGVHD grade II–IV | 27.9% [18.6–37.9] | 21.4% [15.4–28.1] | 0.25 |
| aGVHD grade III–IV | 17.8% [10.5–26.7] | 9% [5.3–14] | 0.042 |
| cGVHD | 17.2% [9.6–26.6] | 32.5% [25.1–40.2] | 0.012 |
| Extensive cGVHD | 5% [1.6–11.4] | 16.9% [11.2–23.5] | 0.011 |
- aGVHD = acute graft-versus-host disease; cGVHD = chronic graft-versus-host disease; GRFS = GVHD-free and relapse-free survival; Haplo = haploidentical; HCT = hematopoietic cell transplantation; LFS = leukemia-free survival; MSD = matched sibling donor; NRM = nonrelapse mortality; OS = overall survival; RI = relapse incidence.
In the multivariate analysis, the risk of NRM was significantly higher in haploHCT in comparison with MSD-HCT (HR = 2.03; 95% CI: 1.03–4.02; P = 0.042), which translated into significantly lower chance of OS (HR = 1.72; 95% CI: 1.15–2.58; P = 0.009). No significant effect of the type of donor was observed in other transplant outcomes including RI, LFS, and GRFS, as well as the risk of aGVHD and cGVHD (Table 4). Among other prognostic factors, KPS ≥90 was associated with improved OS, LFS, and GRFS while the reduced risk of RI, NRM, aGVHD, and cGVHD. AlloHCT performed in second or subsequent relapse as compared to primary refractory ALL or first relapse were associated with increased risk of RI and decreased chance of OS, LFS, and GRFS (Table 4).
| Relapse | NRM | LFS | OS | GRFS | aGVHD II–IV | cGVHD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| HaploHCT vs MSD-HCT | 0.97 (0.62–1.52) | 0.89 | 2.03 (1.03–4.02) | 0.042 | 1.22 (0.84–1.78) | 0.3 | 1.72 (1.15–2.58) | 0.009 | 1.31 (0.88–1.94) | 0.18 | 1.85 (0.99–3.46) | 0.054 | 0.56 (0.26–1.21) | 0.14 |
| Age (per 10 y) | 1.03 (0.88–1.19) | 0.74 | 1.05 (0.83–1.33) | 0.69 | 1.04 (0.92–1.18) | 0.51 | 1.09 (0.95–1.26) | 0.2 | 0.92 (0.81–1.05) | 0.23 | 0.91 (0.73–1.12) | 0.36 | 0.99 (0.78–1.26) | 0.93 |
| ALL subtype | ||||||||||||||
| Ph (–) B-ALL (reference) | ||||||||||||||
| Ph (+) B-ALL | 0.78 (0.44–1.39) | 0.4 | 0.79 (0.34–1.84) | 0.58 | 0.81 (0.5–1.3) | 0.38 | 0.89 (0.52–1.52) | 0.68 | 0.76 (0.46–1.25) | 0.28 | 0.85 (0.38–1.89) | 0.69 | 1.93 (0.77–4.8) | 0.16 |
| T-ALL | 0.77 (0.48–1.25) | 0.29 | 0.64 (0.31–1.35) | 0.24 | 0.74 (0.49–1.12) | 0.15 | 0.89 (0.56–1.41) | 0.61 | 0.63 (0.42–0.97) | 0.036 | 0.5 (0.27–0.93) | 0.029 | 1.4 (0.64–3.09) | 0.4 |
| ALL status at transplantation | ||||||||||||||
| Refractory (reference) | ||||||||||||||
| First relapse | 1.27 (0.82–1.97) | 0.29 | 0.84 (0.43–1.65) | 0.61 | 1.12 (0.77–1.63) | 0.54 | 0.98 (0.65–1.48) | 0.92 | 0.94 (0.64–1.37) | 0.74 | 1.03 (0.56–1.86) | 0.93 | 1.06 (0.53–2.11) | 0.87 |
| Second relapse | 2.07 (1.23–3.47) | 0.006 | 0.86 (0.36–2.03) | 0.72 | 1.61 (1.03–2.51) | 0.035 | 1.8 (1.11–2.91) | 0.017 | 1.36 (0.85–2.17) | 0.2 | 0.91 (0.42–1.97) | 0.81 | 0.7 (0.24–2.04) | 0.52 |
| Female donor to male patient | 0.86 (0.56–1.32) | 0.48 | 0.93 (0.49–1.77) | 0.84 | 0.88 (0.61–1.25) | 0.47 | 0.95 (0.65–1.41) | 0.81 | 0.8 (0.55–1.17) | 0.25 | 1.42 (0.81–2.5) | 0.22 | 1.48 (0.79–2.77) | 0.22 |
| KPS≥90 | 0.51 (0.34–0.76) | 0.001 | 0.28 (0.15–0.51) | < 0.0001 | 0.43 (0.31–0.6) | < 0.0001 | 0.36 (0.25–0.51) | < 0.0001 | 0.44 (0.31–0.61) | < 0.0001 | 0.55 (0.32–0.96) | 0.035 | 0.43 (0.22–0.83) | 0.012 |
| RIC vs MAC | 0.98 (0.59–1.61) | 0.93 | 0.71 (0.3–1.66) | 0.43 | 0.89 (0.58–1.37) | 0.59 | 0.71 (0.44–1.14) | 0.16 | 1.02 (0.65–1.6) | 0.95 | 0.69 (0.31–1.55) | 0.37 | 0.64 (0.27–1.48) | 0.29 |
| TBI vs CT | 0.71 (0.46–1.08) | 0.11 | 1.05 (0.54–2.05) | 0.89 | 0.79 (0.55–1.13) | 0.2 | 0.84 (0.56–1.24) | 0.37 | 0.85 (0.58–1.23) | 0.38 | 1.15 (0.63–2.13) | 0.65 | 0.84 (0.42–1.66) | 0.62 |
| PB vs BM | 1.08 (0.65–1.79) | 0.76 | 1.08 (0.5–2.34) | 0.84 | 1.06 (0.7–1.62) | 0.78 | 1.15 (0.72–1.83) | 0.55 | 1.35 (0.86–2.11) | 0.2 | 1.31 (0.63–2.71) | 0.47 | 1.79 (0.77–4.17) | 0.18 |
- aGVHD = acute graft-versus-host disease; ALL = acute lymphoblastic leukemia; BM = bone marrow; cGVHD = chronic graft-versus-host disease; CT = chemotherapy; GRFS = GVHD-free and relapse-free survival; Haplo = haploidentical; HCT = hematopoietic cell transplantation; KPS = Karnofsky performance status; LFS = leukemia-free survival; MAC = myeloablative conditioning; MSD = matched sibling donor; NRM = nonrelapse mortality; OS = overall survival; PB = peripheral blood; Ph = Philadelphia; RIC = reduced-intensity conditioning; TBI = total body irradiation.
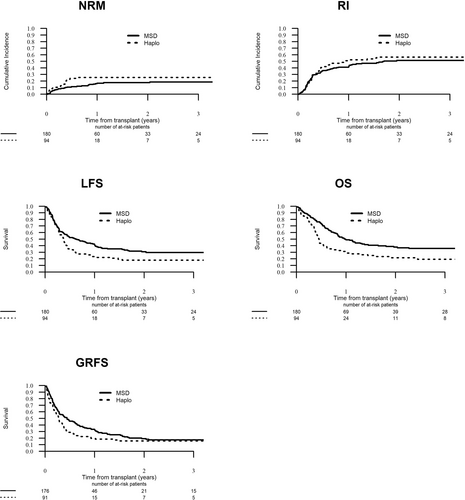
Transplantation outcome—NRM, RI, LFS, OS, and GRFS of allogeneic stem cell transplantation from Haplo donors and MSD in patients with relapsed/refractory acute lymphoblastic leukemia. GRFS = GVHD-free, relapse-free survival; GVHD = graft-versus-host disease; Haplo = haploidentical; LFS = leukemia-free survival; MSD = matched sibling donors; NRM = nonrelapse mortality; OS = overall survival; RI = relapse incidence.
DISCUSSION
In this study, we compared outcomes of haploHCT and MSD-HCT in patients with primary R/R ALL and showed the superiority of MSD-HCT over haploHCT in terms of NRM and OS. The incidence of aGVHD grade III–IV was higher in haploHCT, while the incidence of extensive cGVHD, was higher in MSD-HCT. To our knowledge, this is the first study comparing alloHCT from haploHCT and MSD-HCT in this setting.
Despite improvements in first-line therapies for adults with ALL, still a majority of patients with high-risk disease experience relapse or primary refractoriness. The prognosis of adult patients with R/R ALL is dismal. According to PETHEMA (Programa Español de Tratamiento en Hematologia) Group, the median OS for ALL patients after the first relapse is only 4.5 months with a 5-year OS rate of 10%.23 Furthermore, the 1-year survival rate of ALL patients after second salvage chemotherapy is only 18%.24 The results of alloHCT in patients with R/R acute leukemias are inferior compared to transplantations performed in CR; however, most studies in this area were focused on patients with acute myeloid leukemia, and only a few analyses included patients with ALL. In the largest so far study by Duval et al, 582 patients undergoing alloHCT with R/R ALL were included with a 16% OS rate at 3 years.25. In another study, Pavlů et al assessed alloHCT in primary refractory ALL. In that analysis, the probability of OS for the entire group was 36% at 2 years and 23% at 5 years.4 The authors demonstrated the superiority of TBI over other conditioning regimens in relation to OS and LFS. They also showed the benefit of a female donor to male recipient combination in terms of LFS.4
The success of alloHCT depends on both the antileukemic effect of the conditioning regimen and the GVL reaction.26-28 In patients with ALL, the relevance of GVL reaction is supported by a very strong association between the incidence of GVHD and relapse.28 Both aGVHD and cGVHD are associated with a reduced risk of relapse.28 The GVL effect may potentially be enhanced by donor/recipient HLA disparity. Fan et al reported data from 2 prospective trials including 335 patients with Ph-negative B-ALL treated with either MSD-HCT or haploHCT, mostly in CR.29 The incidence of MRD persistence or relapse at 3 years was significantly reduced for those undergoing haploHCT (27% versus 43%; P = 0.003). A similar comparison, restricted to patients with ALL in CR1 or CR2, was performed by our study group.10 LFS and OS rates for 413 recipients of haploHCT and 1891 patients treated with MSD-HCT were similar, however, the risk of relapse was reduced for haploHCT (HR = 0.66; P = 0.004) without significant effect on NRM, LFS, and OS. HaploHCT compared with MSD-HCT was associated with increased risk of both grade 2–4 and grade 3–4 aGVHD while the reduced risk of extensive cGVHD.10 HaploHCT was also compared with either 10/10 MUD or unrelated donor with a single HLA disparity. In 2 registry-based analyses, the outcomes were comparable for all 3 donor types.11, 30 Kharfan-Dabaja et al compared haploHCT with MUD-HCT as a second alloHCT in patients with ALL achieving CR presecond transplant.31 The authors did not show a significant difference in terms of OS and LFS between the 2 donor types.
Results of the above-cited studies suggest a stronger GVL effect after haploHCT compared with MSD-HCT, but not MUD-HCT. However, in this study, we could not confirm the benefit of haploHCT. The incidences of relapse after MSD-HCT and haploHCT were comparable, suggesting that the GVL effect driven by HLA incompatibility may be insufficient in the presence of a very high tumor burden at transplantation as in the case of R/R ALL. In this context, a reasonable strategy would be to attempt to reduce the tumor mass, and optimally achieve CR before transplantation. In the modern era, this goal may be reached by the use of monoclonal antibodies, either bispecific T-cell engager (blinatumomab) or immunotoxin (inotuzumab ozogamycin) for B-ALL, as well as a deoxyguanosine analog—nelarabine for T-ALL.32-34
Although the results of our study suggest that MSD should still be considered a preferable donor for patients with R/R ALL, it should be noted that unmanipulated haploHCT has been widely implemented in a relatively recent period and the results tend to improve over time. According to a recent retrospective study by our group focusing on adults with ALL, NRM and overall mortality decreased by more than 50% for haploHCT performed between 2016 and 2018 compared with the 2011–2015 period.35 We also reported better results for haploHCT in ALL using PTCy compared to ATG as a backbone of immunosuppression.18 Furthermore, the outcomes were better for bone marrow compared with peripheral blood as a source of stem cells, as a consequence of a significantly reduced risk of both aGVHD and cGVHD for bone marrow grafts.36 Finally, the use of myeloablative conditioning based on TBI was associated with superior LFS compared with chemotherapy-based regimens.27 It should be noted that in this study, among recipients of haploHCT, 11.7% of patients were treated with immunosuppressive protocols other than PTCy, 56.4% of patients received peripheral blood grafts, and only 31.9% of patients were treated with TBI. Still in agreement with previous publications in patients with acute leukemia and those with ALL patients in CR matched sibling is the best donor due to low incidence of NRM.10, 38
Furthermore, results of MDS-HCT in ALL are improving as well, mainly due to a reduction in NRM most probably a consequence of improved supportive care as we and others have shown.3, 37, 38 The recent progress in therapeutic availability of novel compounds for cGVHD with 3 compounds (ibrutinib, ruxolitinib, and belumosudil) approved by the US Food and Drug Administration (FDA) in the last 5 years and the increasing use of PTCy for GVHD prophylaxis, holds potential for further improvements in outcomes of MSD-HCT in ALL.9, 39 Altogether, with optimization of the transplant procedure from both haploidentical and sibling donors, the role of haploHCT in R/R ALL may still be of value, which, however, requires re-evaluation in the future.
Until recently, alloHCT was considered the only curative approach for patients with R/R ALL. In the modern era of treatment, chimeric antigen receptor (CAR) T-cells appears an attractive alternative. The use of tisagenlecleucel in children and young adults with B-ALL is associated with an 82% response rate and 55% probability of OS at 5 years.40, 41 Response rate after the treatment with brexucaptagene autoleucel for adults with R/R ALL is 71% with 25 months median OS.42 On the one hand, wide application of CAR T-cells may diminish the need for alloHCT, on the other, alloHCT may still be considered as consolidation after CAR T-cells in order to reduce the risk of relapse.
Our study has some important limitations related to its retrospective nature. In particular, detailed data regarding clinical characteristics (percentage of bone marrow blasts, presence of extramedullary lesions), details on molecular features of the disease, and data on pre- and post-transplant pharmacological interventions were lacking. These variables of potential prognostic value could not be included in the analysis. In addition, the MSD-HCT and haploHCT groups differed in several parameters including disease status, conditioning and graft sources that all were included in the Cox model. Nevertheless, we were able to demonstrate that approximately 20% of patients with R/R ALL may be rescued with haploHCT and that the proportion is even higher (above 35%) after MSD-HCT. In both cases, however, the incidence of disease progression exceeds 50% being the major cause of treatment failure. Further attempts should be focused on the optimal use of modern treatment options (monoclonal antibodies, CAR T-cells) as either pre- or post-transplant interventions aiming to achieve and maintain CR in the context of alloHCT.
ACKNOWLEDGMENTS
We thank all the EBMT centers and national registries for contributing patients to this study (Suppl. Appendix – contributing centers). We also thank the data managers for their excellent work, and the patients who provided data.
AUTHOR CONTRIBUTIONS
AN wrote the study synopsis and abstract, designed the study, interpreted the data, and edited the article. ML designed the study, performed the statistical analyses, interpreted the data, and edited the article. RS wrote the article. MM designed the study, interpreted the data, and edited the article. EB, PZ, SG, and FC interpreted the data and reviewed the article. PP, MA, IYA, AK, EA, HO, AR, ZNO, and JS reviewed the article and provided clinical data. All authors approved the final version of the article.
DISCLOSURES
The authors declare that they have no relevant conflict of interest and no competing financial interests.