High Molecular and Cytogenetic Risk in Myelofibrosis Does Not Benefit From Higher Intensity Conditioning Before Hematopoietic Cell Transplantation: An International Collaborative Analysis
NG, RBS, BLS, and NK have contributed equally to this work.
Abstract
There is no direct evidence to recommend specific conditioning intensities in myelofibrosis undergoing allogeneic hematopoietic cell transplantation, especially in the molecular era. We aimed to compare outcomes of reduced intensity (RIC) or myeloablative conditioning (MAC) transplantation in myelofibrosis with molecular information. The study included 645 genetically annotated patients (with at least driver mutation status available), of whom 414 received RIC and 231 patients received MAC. The median follow-up time from transplantation was 6.0 years for RIC and 9.4 years for MAC. The 6-year overall survival rates for RIC and MAC were 63% (95% confidence interval [CI], 58%-68%) and 59% (95% CI, 52%-66%; P = 0.34) and progression-free survival was 52% (95% CI, 47%-57%) and 52% (95% CI, 45%-59%; P = 0.64). The 2-year cumulative incidence of nonrelapse mortality was 26% (95% CI, 21%-31%) for RIC and 29% (95% CI, 23%-34%) for MAC (P = 0.51). In terms of progression/relapse, the 2-year cumulative incidence was 10% (95% CI, 5%-19%) for RIC and 9% (95% CI, 4%-14%) for MAC (P = 0.46). Higher intensity conditioning did not seem to improve outcomes for higher-risk disease, according to mutational, cytogenetic, and clinical profile. In contrast, patients with reduced performance status, matched unrelated donors, and ASXL1 mutations appeared to benefit from RIC in terms of overall survival.
INTRODUCTION
Myelofibrosis is a chronic myeloproliferative neoplasm developing either de novo (as primary myelofibrosis) or evolving from essential thrombocytosis or polycythemia vera (as secondary or post-ET/PV myelofibrosis).1 Although significant improvement has been achieved over recent years in the symptomatic treatment of myelofibrosis by incorporating Janus-Kinase-inhibition,2, 3 and more recently targeted therapies,4 allogeneic stem cell transplantation remains the only potentially curative option.5
One key aspect of this complex treatment platform6 is the type and intensity of the conditioning regimen prior to the actual stem cell infusion.7 Over the years, several studies established the feasibility of both myeloablative (MAC) and reduced intensity (RIC) regimens.7-12 In unadjusted comparisons in a recent retrospective study from the European Society for Blood and Marrow Transplantation (EBMT), results for survival were comparable between RIC and MAC, while relapse rates appeared to be higher after RIC.13 Furthermore, no difference was observed between RIC regimens consisting of busulfan-fludarabine and fludarabine-melphalan in the RIC setting, whereas other studies indicated better survival outcomes but higher relapse rates for RIC using busulfan-fludarabine.14, 15 Other studies showed no significant benefit of one RIC regimen over another.15-18 Still, it is unclear whether higher intensity conditioning may overcome onset worse prognosis of higher-risk disease.7, 19
However, most of the existing analyses in myelofibrosis provided only very limited information when comparing intensities according to different clinical but even more molecular and cytogenetic risk categories, which has recently extended and refined the understanding of prognosis in the nontransplant as well as in the transplant setting.20-23
Thus, the present study aimed to evaluate outcome after different conditioning intensities according to clinical and molecular information in patients with myelofibrosis.
METHODS
Patients
A total of 645 patients with primary or post-ET/PV myelofibrosis undergoing first allogeneic stem cell transplantation were included. Patient data were collected from the University Medical Center Hamburg-Eppendorf (Hamburg, Germany), the West German Cancer Center (Essen, Germany), Hôpital Saint-Louis (Paris, France), the Fred Hutchinson Cancer Research Center (Seattle, WA, USA), and Hannover Medical School (Hannover, Germany). Patients with myelofibrosis in progression to acute leukemia were excluded. Patients had to receive transplant before 2021. All relevant clinical and transplant-specific variables, and samples for sequencing and cytogenetic analyses were collected at time of transplant. Reduced intensity conditioning prior to transplantation was defined by using busulfan-fludarabine (given as 10 mg/kg bodyweight and 150 or 180 mg/m2), fludarabine-melphalan (given as 150 and 140 mg/m2), sequential fludarabine-amsacrine-based, treosulfan-fludarabine (given as 10 and 150 mg/m²), or 2-Gy total body irradiation/fludarabine regimen (150 mg/m2). The study was conducted in accordance with the Declaration of Helsinki.
Mutational and cytogenetic analyses
Bone marrow or peripheral blood samples were obtained before transplantation and mutations were detected using next-generation sequencing as previously described.24-26 The following myelofibrosis-associated genes were considered: JAK2, CALR, MPL, ASXL1, IDH1/2, CBL, DNMT3A, TET2, SF3B1, SRSF2, U2AF1, EZH2, TP53, NRAS, KRAS, RUNX1, and FLT3. High-risk molecular genetic (HMR) was defined as positive for IDH1/2, SRSF2, AXSL1, or EZH2.27 Cytogenetic analysis and reporting were performed according to the International System for Human Cytogenetic Nomenclature criteria using standardized techniques; and cytogenetic risk was categorized in accordance with Tefferi et al.28
End points
The primary end point of the study was overall survival, which was defined as the time from transplantation to death from any cause or last follow-up. Death from any cause was considered an event. Surviving patients were censored at last follow-up. Secondary end points were nonrelapse mortality, progression-free survival, cumulative incidence of relapse. Progression-free survival was defined as time from transplantation to either relapse or death from any cause. Nonrelapse mortality was defined as death from any cause as a cumulative incidence estimate, with relapse as competing risk; and relapse was summarized by cumulative incidence estimate with nonrelapse mortality as the competing event.
Statistical analysis
This is a retrospective cohort study comparing outcomes after RIC versus MAC allogeneic hematopoietic cell transplantation using related and unrelated donors for patients with myelofibrosis. Eligible patients were stratified according to RIC versus MAC.
Probabilities of survival were calculated using Kaplan-Meier estimates. Probabilities of nonrelapse mortality and relapse were calculated by cumulative incidence function accounting for competing risks. Risk ratios (RRs) were obtained for subgroup analysis and a forest plot was used to depict estimates with corresponding 95% confidence intervals (CIs). Next, multivariable analyses were performed to evaluate associations among patient-related, disease-related, donor-related, and transplantation-related variables and outcomes of interest using a Cox proportional hazards regression model for survival and Fine and Gray for competing risk outcomes. Hazard ratios (HRs) with corresponding 95% CI were calculated for risk estimation. Backward stepwise selection was used to identify significant covariates that influenced outcomes of RIC versus MAC. Comparisons with P <0.05 were considered significantly different. The proportional hazards assumption for Cox regression was tested using Schoenfeld residuals. Covariates violating the proportional hazards assumption were otherwise added as time-dependent covariates. In addition, a Dependent Dirichlet Process model for survival analysis data was developed.29 A major feature of the proposed approach is that there is no necessity for resulting survival curve estimates to satisfy the ubiquitous proportional hazards assumption. In the case of missing information, multiple imputation was used.30 All analyses were performed using R statistical software version 4.0.5.
RESULTS
Patients
Patient and transplant characteristics are shown in Table 1. Of all 645 patients, 414 myelofibrosis patients received RIC and 231 patients received MAC. Median age in the RIC group was higher (58 versus 54 years; P < 0.001). Forty percent in the RIC group and 46% in the MAC group were female patients (P = 0.15). Distribution of risk categories according to the DIPSS31 was significantly different between both groups (RIC versus MAC, P < 0.001): 5% versus 17% for low risk, 29% versus 35% for intermediate-1 risk, 50% versus 43% for intermediate-2 risk, and 16% versus 5% for high risk. Patients in the RIC group had lower performance status (P = 0.03). However, there was an interaction between performance status and age between the groups, with MAC showing more patients at younger age with lower Karnofsky performance status of <90% (interaction P = 0.04).
| Characteristic | RIC (n = 414) | MAC (n = 231) | P |
|---|---|---|---|
| Age at HCT in years, median (range) | 58 (18–78) | 54 (21–71) | <0.001 |
| Female sex, n (%) | 165 (40) | 106 (46) | 0.15 |
| Diagnosis, n (%) | 0.001 | ||
| PMF | 290 (70) | 133 (58) | |
| SMF | 124 (30) | 98 (42) | |
| Transfusion dependence | 202 (49) | 127 (55) | 0.16 |
| Blood levels, median (range) | |||
| Hemoglobin, g/dL | 9.5 (5.6–17.6) | 9.9 (5.6–16.0) | 0.12 |
| Circulating blasts, % | 1 (0–19) | 1 (0–19) | 0.51 |
| Platetels, ×106/L | 144 (5–2437) | 165 (4–3506) | 0.18 |
| Leukocytes, ×106/L | 8.1 (0.6–168.8) | 8.6 (0.4–93.7) | 0.70 |
| Karnofsky performance status, n (%) | 0.03 | ||
| 90%–100% | 244 (59) | 159 (69) | |
| <90% | 170 (41) | 72 (31) | |
| Driver mutation genotype, n (%) | 0.07 | ||
| CALR | 78 (19) | 52 (23) | |
| MPL | 25 (6) | 4 (2) | |
| JAK2 | 237 (57) | 135 (58) | |
| Triple negative | 74 (18) | 40 (18) | |
| ASXL1 mutation presenta | 119 (29) | 50 (29) | 0.97 |
| HMR presentb | 148 (41) | 59 (38) | 0.55 |
| DIPSS, n (%) | <0.001 | ||
| Low | 21 (5) | 38 (17) | |
| Intermediate-1 | 118 (29) | 82 (35) | |
| Intermediate-2 | 212 (50) | 100 (43) | |
| High | 66 (16) | 11 (5) | |
| Cytogenetic risk, n (%)c | 0.31 | ||
| Favorable | 190 (73) | 81 (68) | |
| Unfavorable | 49 (19) | 31 (26) | |
| VHR | 20 (8) | 8 (7) | |
| Time to HCT in years, median (range) | 2.2 (0.01–47.3) | 1.4 (0.02–26.1) | 0.08 |
| Donor type | <0.001 | ||
| Matched related | 102 (25) | 90 (38) | |
| Matched unrelated | 220 (53) | 102 (44) | |
| Mismatched related | 2 (1) | 5 (2) | |
| Mismatched unrelated | 90 (21) | 34 (15) | |
| Conditioning regimen | <0.001 | ||
| Flamsa-based | 36 (9) | 0 | |
| BuFlu | 261 (63) | 8 (4) | |
| TreoFlu | 12 (3) | 32 (14) | |
| TBICy | 0 | 14 (6) | |
| FluMel | 74 (18) | 0 | |
| BuCy | 0 | 136 (59) | |
| Other | 31 (8) | 41 (18) | |
| Cell source | 0.16 | ||
| Peripheral blood | 403 (97) | 220 (95) | |
| Bone marrow | 11 (3) | 11 (5) |
- aFrom 579 patients (407 for RIC and 172 for MAC).
- bFrom 517 patients; the number of patients with HMR other than ASXL1 according to conditioning intensity (RIC vs MAC) was 33 vs 6 for SRSF2, 10 vs 3 for IDH1, 12 vs 2 for IDH2, 14 vs 2 for EZH2.
- cFrom 379 patients; cytogenetic risk stratification according to Tefferi et al.28
- Bu = busulfan; Cy = cyclophosphamide; Flamsa = fludarabine+amsacrine; Flu = fludarabine; HCT = hematopoietic cell transplantation; HMR = high molecular risk; MAC = myeloablative conditioning; Mel = melphalan; RIC = reduced intensity conditioning; TBI = total body irradiation.
Five hundred seventeen patients had full information on HMR status in accordance with Vannucchi et al,27 of whom 41% in the RIC group and 38% in the MAC group showed present HMR at time of transplantation. Risk stratification according to the mutation-enhanced IPSS (MIPSS70) for the RIC and MAC groups was 1% and 8% (low risk), 41% and 48% (intermediate risk), and 58% and 44% (high risk; P < 0.001).
Transplantation and conditioning
The median time in months between diagnosis of myelofibrosis and transplantation was 2.2 years (range, 0.01–47.3 years) in the RIC group and 1.4 years (range, 0.02–26.1 years) in the MAC group (P = 0.08). Distribution of donor relation was significantly different between the groups (P < 0.001), with RIC having more matched unrelated donor transplants, whereas MAC had more matched related transplants (Table 1). Serostatus for cytomegalovirus for transplant recipient/donor were different between the groups (P = 0.04). More donor/recipient pairs were both seropositive for cytomegalovirus in the RIC group (50%) compared with the MAC group (38%). The main regimen in the RIC group was busulfan-fludarabine and the main regimen in the MAC group was busulfan-cyclophosphamide. Most transplants in both conditioning intensity groups (97% for RIC and 95% for MAC) had peripheral blood as graft source.
Within-group outcomes: RIC
In terms of overall survival, several clinical and molecular variables influenced outcome (Table 2). For clinical variables, significant effects were seen for age, Karnofsky performance status, constitutional symptoms, and a mismatched unrelated donor allograft. For molecular variables, driver mutation genotype and ASXL1 status were associated with outcome. For driver mutation status, 6-year overall survival was 81% (95% CI, 71%-91%) for CALR, 58% (95% CI, 51%-65%) for JAK2, 83% (95% CI, 67%-99%) for MPL, and 55% (95% CI, 44%-66%) for triple negative patients (Figure 1A). No significant difference in the outcome was observed for CALR-type 1 versus CALR-type 2 mutations (P = 0.59) nor for the presence of HMR versus absence of HMR (Figure 1B). No significant effect for individual HMR mutations (except for ASXL1) nor for number of HMR mutations was observed. For ASXL1 mutated patients, 6-year overall survival was 54% (95% CI, 44%-64%) compared with 69% (95% CI, 63%-75%) for unmutated patients. Of note, from 10 IDH1-mutated patients, only 1 patient died resulting in 90% 6-year survival rate. No significant effect was observed for currently established cytogenetic risk categories, and the 6-year overall survival was 61% (95% CI, 54%-68%) for favorable risk, 60% (95% CI, 46%-74%) for unfavorable risk, and 65% (95% CI, 32%-98%) for very high-risk categories (P = 0.68). Results were similar for progression-free survival (Table 2).
| RIC | MAC | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P | HR | 95% CI | P |
| Overall survival | ||||||
| Age, y | 1.03 | 1.01-1.05 | 0.005 | 1.02 | 0.99-1.05 | 0.07 |
| Sex | ||||||
| Male | Reference | |||||
| Female | 0.95 | 0.68-1.34 | 0.80 | 0.64 | 0.40-1.01 | 0.06 |
| Diagnosis | ||||||
| PMF | Reference | |||||
| SMF | 1.04 | 0.73-1.48 | 0.19 | 1.32 | 0.86-2.02 | 0.20 |
| Driver mutation | ||||||
| CALR | Reference | |||||
| JAK2 | 2.52 | 1.44-4.29 | 0.001 | 1.79 | 0.98-3.28 | 0.06 |
| MPL | 0.88 | 0.29-2.67 | 0.82 | 1.73 | 0.01-NA | 0.99 |
| Triple negative | 3.20 | 1.71-5.99 | <0.001 | 2.04 | 1.00-4.15 | 0.05 |
| ASXL1-mutated | 1.64 | 1.14-2.35 | 0.01 | 1.60 | 1.19-2-14 | 0.002 |
| HMR present | 1.21 | 0.92-1.60 | 0.18 | 1.27 | 0.77-2.10 | 0.35 |
| KPS | ||||||
| 90%–100% | Reference | 2.42 | 1.51-3.85 | <0.001 | ||
| <90% | 1.40 | 1.00-2.20 | 0.05 | |||
| Constitutional symptoms | 1.48 | 1.02-2.14 | 0.04 | 1.56 | 0.96-2.53 | 0.07 |
| DIPSS | ||||||
| Low | Reference | |||||
| Intermediate-1 | 1.54 | 0.55-4.37 | 0.41 | 2.06 | 0.84-5.05 | 0.12 |
| Intermediate-2 | 1.99 | 0.72-5.44 | 0.18 | 3.16 | 1.33-7.48 | 0.008 |
| High | 2.76 | 0.97-7.88 | 0.06 | .69 | 0.76-9.56 | 0.12 |
| Cytogenetics | ||||||
| Favorable | Reference | 1.70 | ||||
| Unfavorable | 1.23 | 0.74-2.05 | 0.43 | 1.38 | 0.86-3.25 | 0.11 |
| VHR | 1.03 | 0.47-2.25 | 0.94 | 0.42-4.56 | 0.60 | |
| Donor type | ||||||
| MRD | Reference | |||||
| MUD | 0.95 | 0.62-1.45 | 0.81 | 1.39 | 0.91-2.11 | 0.12 |
| MMRD | 2.54 | 0.34-18.59 | 0.36 | 0.29 | 0.04-2.14 | 0.22 |
| MMUD | 1.91 | 1.21-2.99 | 0.005 | 1.83 | 1.05-3.18 | 0.03 |
| Time to transplant | 1.00 | 0.97-1.03 | 0.94 | 0.97 | 0.92-1.02 | 0.24 |
| Progression-free survival | ||||||
| Age, y | 1.02 | 1.00-1.04 | 0.02 | 1.03 | 1.01-1.06 | 0.006 |
| Sex | ||||||
| Male | Reference | Reference | ||||
| Female | 1.00 | 0.74-1.35 | 0.99 | 0.66 | 0.43-1.01 | 0.06 |
| Diagnosis | ||||||
| PMF | Reference | |||||
| SMF | 1.19 | 0.87-1.62 | 0.27 | 1.43 | 0.96-2.11 | 0.08 |
| Driver mutation | ||||||
| CALR | Reference | |||||
| JAK2 | 2.52 | 1.57-4.04 | <0.001 | 1.90 | 1.10-3.28 | 0.02 |
| MPL | 0.95 | 0.38-2.37 | 0.91 | 1.73 | 0.01-NA | 0.99 |
| Triple negative | 2.68 | 1.56-4.60 | <0.001 | 1.76 | 0.90-3.46 | 0.09 |
| ASXL1-mutated | 1.52 | 1.10-2.10 | 0.01 | 1.93 | 1.15-3.22 | 0.01 |
| HMR present | 1.15 | 0.86-1.54 | 0.25 | 1.20 | 0.83-1.67 | 0.38 |
| KPS | ||||||
| 90%–100% | Reference | |||||
| <90% | 1.19 | 0.88-1.62 | 0.27 | 2.30 | 1.49-3.55 | <0.001 |
| Constitutional symptoms | 1.30 | 0.94-1.77 | 0.11 | 1.56 | 0.99-2.44 | 0.06 |
| DIPSS | ||||||
| Low | Reference | |||||
| Intermediate-1 | 1.37 | 0.58-3.21 | 0.47 | 0.78 | 0.95-5.02 | 0.07 |
| Intermediate-2 | 1.76 | 0.77-4.01 | 0.18 | 1.18 | 1.47-7.26 | 0.003 |
| High | 2.23 | 0.94-5.32 | 0.07 | 1.41 | 1.38-12.26 | 0.001 |
| Cytogenetics | ||||||
| Favorable | Reference | |||||
| Unfavorable | 1.02 | 0.64-1.63 | 0.94 | 1.37 | 0.76-2.47 | 0.30 |
| VHR | 1.00 | 0.50-1.98 | 0.99 | 0.95 | 0.29-3.09 | 0.93 |
| Donor type | ||||||
| MRD | Reference | |||||
| MUD | 1.05 | 0.72-1.52 | 0.81 | 1.30 | 0.84-2.02 | 0.24 |
| MMRD | 3.78 | 0.91-15.66 | 0.07 | 0.34 | 0.09-2.19 | 0.27 |
| MMUD | 1.91 | 1.27-2.87 | 0.001 | 1.76 | 1.00-3.08 | 0.05 |
| Time to transplant | 1.00 | 0.97-1.02 | 0.82 | 0.99 | 0.96-1.04 | 0.80 |
- CI = confidence interval; DIPSS = Dynamic International Prognostic Scoring System; KPS = Karnofsky performance status; MAC = myeloablative conditioning; MMRD = mismatched related donor; MMUD = mismatched unrelated donor; MRD = matched related donor; MUD = matched unrelated donor; OS = overall survival; PFS = progression-free survival; PMF = primary myelofibrosis; SMF = secondary myelofibrosis; VHR = very high risk.
Within-group outcomes: MAC
In terms of overall survival, results were generally comparable with RIC subgroups, and several clinical and molecular variables influenced outcome (Table 2). For clinical variables, statistically significant effects were seen for DIPSS intermediate-2 risk category (with low risk as reference), Karnofsky performance status, and mismatched unrelated donor allografts, while other variables such as age, constitutional symptoms, and patient sex appeared to affect outcome. For molecular variables, driver mutation genotype and ASXL1 status were also associated with outcome. For driver mutation status, 6-year overall survival was 72% (95% CI, 59%-85%) for CALR, 55% (95% CI, 46%-64%) for JAK2, 100% for MPL, and 55% (95% CI, 40%-70%) for triple negative patients (Figure 1C). No significant difference in the outcome was observed for CALR-type 1 versus CALR-type 2 mutations (P = 0.54) nor for the presence of HMR versus absence of HMR (Figure 1D). No significant effect for individual HMR mutations (except for ASXL1) nor for number of HMR mutations was observed. For ASXL1-mutated patients, 6-year overall survival was 47% (95% CI, 31%-61%) compared with 64% (95% CI, 53%-73%) for unmutated patients. No significant effect was observed for currently established cytogenetic risk categories, and the 6-year overall survival was 63% (95% CI, 53%-73%) for favorable risk, 52% (95% CI, 34%-70%) for unfavorable risk, and 63% (95% CI, 30%-96%) for very high-risk categories (P = 0.57). Results were similar for progression-free survival (Table 2).
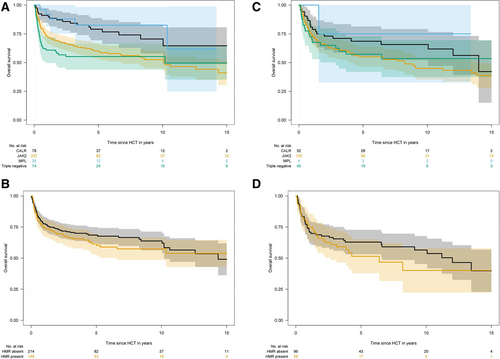
Overall survival according to driver mutation status and high molecular risk status within both conditioning intensity groups. In the RIC group: 6-year overall survival (A) was 81% for CALR, 58% for JAK2, 83% for MPL, and 55% for triple negative patients. No significant difference in outcome was observed for CALR-type 1 versus CALR-type 2 mutations (P = 0.59) nor for presence of HMR versus absence of HMR (B). In the MAC group: 6-year overall survival (C) was 72% for CALR, 55% for JAK2, 100% for MPL, and 55% for triple negative patients. No significant difference in outcome was observed for presence of HMR versus absence of HMR (D). HMR = high molecular risk (as defined by Vannucchi et al27 includes ASXL1, SRSF2, IDH1/2, EZH2); MAC = myeloablative conditioning; RIC = reduced intensity conditioning.
Outcomes for RIC versus MAC
The median follow-up time of survivors from transplantation according to conditioning intensity was 6.0 years (95% CI, 5.1-6.9 years) for RIC and 9.4 years (95% CI, 8.2-10.8 years) for MAC (P < 0.001). The 6-year overall survival rates for RIC and MAC were 63% (95% CI, 58%-68%) and 59% (95% CI, 52%-66%; P = 0.34; Figure 2A). In terms of progression-free survival, 6-year rates were 52% (95% CI, 47%-57%) for RIC and 52% (95% CI, 45%-59%) for MAC (P = 0.64; Figure 2B). Median overall survival and progression-free survival were 12.6 years (95% CI, 8.2-16.9 years) and 6.7 years (95% CI, 4.1-9.4 years) for RIC in comparison with 12.0 years (95% CI, 8.3-15.7 years) and 8.1 years (95% CI, 2.6-13.7 years) for MAC.
The 2-year cumulative incidence of nonrelapse mortality was 26% (95% CI, 21%-31%) for RIC and 29% (95% CI, 23%-34%) for MAC (P = 0.51; Figure 2C). In terms of progression/relapse, the 2-year cumulative incidence was 10% (95% CI, 5%-19%) for RIC and 9% (95% CI, 4%-14%) for MAC (P = 0.46; Figure 2D).
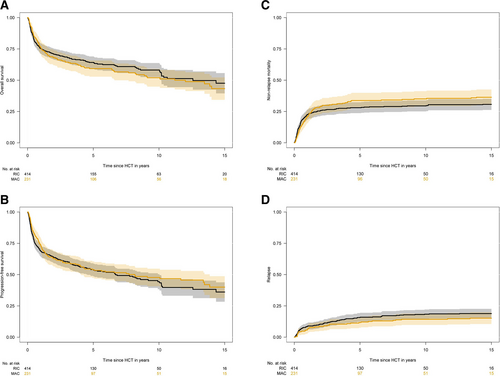
Posttransplant outcomes according to conditioning intensity. The 6-year overall survival (A) rates for RIC and MAC were 63% and 59% (P = 0.34). In terms of progression-free survival (B), 6-year rates were 52% for RIC and 52% for MAC (P = 0.64). The 2-year cumulative incidence of nonrelapse mortality (C) was 26% for RIC and 29% for MAC (P = 0.51). In terms of progression/relapse (D), the 2-year cumulative incidence was 10% for RIC and 9% for MAC (P = 0.46). MAC = myeloablative conditioning; RIC = reduced intensity conditioning.
Clinical-molecular subgroups: RIC versus MAC
In general, the comparison of RIC versus MAC yielded no significant difference in survival for most subgroups in terms of overall survival and progression-free survival. For overall survival, reduced risk for death in favor of RIC appeared to exist for younger patients (RR, 0.68; 0.48-0.97), matched unrelated donor transplants (RR, 0.66; 95% CI, 0.49-0.89), DIPSS intermediate-2 risk at time of transplant (RR, 0.72; 95% CI, 0.54-0.96), Karnofsky performance status <90% (RR, 0.74; 95% CI, 0.55-1.00), and ASXL1-mutations (RR, 0.80; 95% CI, 0.58-1.09); whereas MAC appeared to result in better overall survival for matched donors (RR, 1.71; 95% CI, 0.94-2.53). For performance status and age, an interaction was observed (P = 0.03). In terms of progression-free survival, RIC appeared to improve outcomes for Karnofsky performance status <90% (RR, 0.79; 95% CI, 0.60-1.03), DIPSS intermediate-2 (RR, 0.82; 95% CI, 0.64-1.05). Forest plots for the comparison of RIC versus MAC across subgroups are shown in Figure 3.
Multivariable analysis
We developed a Dependent Dirichlet Process model for survival analysis data. A major feature of the proposed approach is that there is no necessity for resulting survival curve estimates to satisfy the ubiquitous proportional hazards assumption. After multivariable adjustment including DIPSS, age, donor relation, center effect, driver mutation and HMR status, and type of disease (Table 3), no significant difference regarding all end points of interest, including overall survival, progression-free survival, nonrelapse mortality, and incidence of relapse was found. For overall survival, the comparison yielded an HR of 1.20 (95% CI, 0.71-2.22; P = 0.30).
| Factor | HR | 95% CI | P |
|---|---|---|---|
| Overall survival | |||
| RIC | Reference | ||
| MAC | 1.20 | 0.71-2.22 | 0.30 |
| Progression-free survival | |||
| RIC | Reference | ||
| MAC | 1.13 | 0.80-1.58 | 0.49 |
| Nonrelapse mortality | |||
| RIC | Reference | ||
| MAC | 1.24 | 0.82-1.87 | 0.31 |
| Relapse | |||
| RIC | Reference | ||
| MAC | 0.80 | 0.35-1.55 | 0.61 |
- Included variables for adjustment: age, DIPSS, driver mutation and high molecular risk status, type of disease, center, and donor type.
- CI = confidence interval; DIPSS = Dynamic International Prognostic Scoring System; HR = hazard ratio; MAC = myeloablative conditioning; RIC = reduced intensity conditioning.
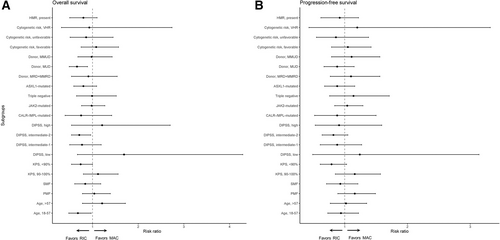
Forest plot and corresponding risk ratios of overall survival (A) and progression-free survival (B) in clinical and molecular subgroups. Risk ratio below 1.0 favors RIC and risk ratio above 1.0 favors MAC. DIPSS = Dynamic International Prognostic Scoring System; HMR = high molecular risk (as defined by Vannucchi et al27 includes ASXL1, SRSF2, IDH1/2, EZH2); KPS = Karnofsky performance status; MAC = myeloablative conditioning; MMRD = mismatched related donor; MMUD = mismatched unrelated donor; MRD = matched related donor; MUD = matched unrelated donor; PMF = primary myelofibrosis; RIC = reduced intensity conditioning; SMF = secondary myelofibrosis; VHR = very high risk according to cytogenetics (Tefferi et al28).
DISCUSSION
Despite advances in therapeutic options for myelofibrosis, allogeneic stem cell transplantation still remains the only curative option, with significantly improved outcome for intermediate-2 and high-risk patients according to DIPSS.5, 32 In patients surviving 2 years after transplantation, 10-year probability of disease-free survival may be 64% and of overall survival even 74%.33 However, treatment-associated morbidity, including the occurrence of late complications, late relapses, infection, and secondary malignancies, highlights the importance of careful patient counseling, screening, and monitoring, especially in the new era of molecular analyses.20, 34
Another key aspect specific to stem cell transplantation and important for patient selection and counseling represents the optimal conditioning intensity before transplantation.35 In this analysis, RIC and MAC were compared with available molecular-genetic information, finding no significant difference favoring one dose-intensity over another for most patients. Of note, higher intensity conditioning for presumed higher-risk disease did not seem to improve outcomes.
Historically, conventional MAC approaches have been considered to be associated with significant toxicity and indeed higher nonrelapse mortality rates. Moreover, the use of RIC platforms extends the potential of the transplant option to older and perhaps more frail candidates, particularly relevant for clinical practice given that the median age of onset of MF is in the sixth and seventh decades. Several small retrospective studies have been published comparing MAC versus RIC approaches (Table 4). For instance, Patriarca et al12 analyzed 100 patients with myelofibrosis who underwent transplantation between 1986 and 2006 on behalf of the Gruppo Italiano Trapianto di Midollo Osseo. Significant improvements in outcomes were seen over time, and the intensity of the conditioning regimen did not significantly influence the transplant outcome.
| Study | OS | NRM | Relapse |
|---|---|---|---|
| EBMT13(RIC vs MAC) | 5 y: 53% vs 51% | 3 y: 32% vs 33% | 3 y: 17% vs 20% |
| Patriarca12(51% RIC) | 3 y: 42% | 3 y: 43% | 2 y: 41% |
| Ditschkowski11(MAC) | 3 y: 38% | 40% | |
| Kröger24(RIC) | 5 y: 56% | 1 y: 21% | 5 y: 25% |
| Kerbauy36(90% MAC) | 5 y: 61% | 5 y: 34% | |
| Jain18(RIC) | 2 y: 61% | ||
| Gupta10(RIC) | 5 y: 47% | 3 y: 22% | 3 y: 47% |
Current study (RIC vs MAC) |
6 y: 63% vs 59% | 2 y: 26% vs 29% | 2 y: 10% vs 9% |
- EBMT = European Society for Blood and Marrow Transplantation; MAC = myeloablative conditioning; NRM = nonrelapse mortality; OS = overall survival; PFS = progression-free survival; RIC = reduced intensity conditioning.
Larger comparisons of conditioning intensities and regimens have recently been undergone. In an analysis from the EBMT of 2224 patients transplanted between 2000 and 2014,13 65% received RIC and 35% MAC. Total body irradiation-based MAC was applied in 17% of patients. Median follow-up time was significantly shorter compared to ours, with 4 years in both RIC and MAC groups. Outcome was similar for both intensities, while relapse rates appeared to be higher after RIC, being 23% at 5 years, which was slightly higher compared with our RIC group.
Regarding conditioning regimens, most of the early MAC platforms consisted of TBI with or without high-dose cyclophosphamide, showing early toxicity and higher treatment-associated morbidity and mortality. One study in 289 patients, of whom 79% received MAC (mainly busulfan-cyclophosphamide and TBI with/without cyclophosphamide) showed +100 days transplant-related mortality of 18% for those undergoing transplantation from an HLA-matched sibling and 35% for those receiving unrelated donor transplantation.17 Another study in 104 patients resulted in 5-year nonrelapse mortality of 34%.36 The estimated 5-year survival rate was 61% for the entire cohort. Patients receiving MAC showed significantly higher survival (68%). More recently, in the EBMT study, no survival difference was seen comparing the most frequent RIC regimens (busulfan-fludarabine versus fludarabine-melphalan versus other) or any of the most frequent MAC regimens (busulfan-cyclophosphamide versus busulfan-fludarabine versus other).13 In contrast, a recent study from CIBMTR demonstrated that the choice of conditioning regimen significantly influences the outcomes.14 The results favored busulfan-fludarabine-based conditioning in the MAC and RIC setting. In our analysis, preliminary comparison of regimens suggested a slight benefit for busulfan-fludarabine RIC regimen, while for MAC, treosulfan-fludarabine appeared to be associated with worse outcome when compared with busulfan-cyclophosphamide or TBI-based regimen. However, it needs to be highlighted that the rationale of this study was dose intensity, while adjusted comparisons of regimens are planned to be undergone in future studies.
All in all, limitations of both historical and rather recent studies were the limited quality of data regarding risk stratification and especially molecular information. As driver and nondriver mutations continue to play an increasing role in diagnosis and risk stratifying patients before transplant and at time of transplant,37 evaluating different transplant platforms with respect to molecular-genetic information is crucial for current counseling of patients, transplant as well as nontransplant physicians. This is especially important when considering the hypothesis that more intensive treatment would be needed for higher-risk disease to eradicate tumor load and aggressiveness. Our analysis showed comparable outcomes of driver mutations in both RIC and MAC transplants, with CALR and MPL showing significantly better survival compared with CALR/ MPL-unmutated genotypes. For CALR types, our analysis confirmed no significant difference between type 1 versus type 2 mutations in both RIC (P = 0.59) and MAC (P = 0.54). For nondriver mutations, onset worse prognosis for present HMR as suggested by nontransplant risk stratifications did not seem to be improved by higher intensity conditioning strategies. Previous reports showed particular relevance of ASXL1 mutations in the nontransplant but also in the transplant setting, showing significantly worse survival in comparison to absence of ASXL1. Here, we observed that RIC appeared to reduce the risk for overall mortality in ASXL1-mutated patients. However, it should be noted that ASXL1 status was not available in all patients, whereas overall frequencies of this mutations were similar between both conditioning groups (29%, respectively). Efforts from our international collaboration are ongoing to include more patients with molecular information to minimize selection bias and to specifically characterize the definite effect of ASXL1 in the transplant setting.
Limitations of the present study are those inherent to retrospective analyses, which are prone to bias owing to different center practices and environments, a lack of transparency for physician choice of regimen intensity, and incomplete data on the patient level. We did not observe a significant difference in overall and progression-free survival between matched related and matched unrelated transplants, and 2-year nonrelapse mortality was 20% versus 25% (P = 0.34). This is in line with previous reports from EBMT and others. We acknowledge that more patients in the RIC cohort received HLA-mismatched unrelated donor transplants and impact of donor type may have been influenced by selection bias and center experience, also confounding the comparison with the same patients receiving MAC. Moreover, time of relapse/progression documentation and time of detection may differ between the centers. Last, the inclusion of patients with molecular information may have introduced selection bias due to center effect and its implementation of molecular monitoring.
In conclusion, RIC and MAC showed, in general, comparable outcome in myelofibrosis with available molecular-genetic information. Especially, higher intensity conditioning transplantation did not seem to improve outcomes of suggested higher-risk disease, whereas patients with reduced performance status, matched unrelated donors and ASXL1 mutations appeared to benefit from RIC.
AUTHOR CONTRIBUTIONS
NG, RBS, BLS, and NK were responsible for the study design, data collection, data analysis, and wrote the manuscript. AB, BC, and VP performed laboratory analyses. TS, CR, CW, FT, MR, BC, and HCR collected data. All authors interpreted the data and contributed to writing the paper.
DISCLOSURES
HCR received consulting and lecture fees from Abbvie, AstraZeneca, Vertex and Merck. HCR received research funding from Gilead Pharmaceuticals. HCR is a co-founder of CDL Therapeutics GmbH. The remaining authors declare no conflict of interest.