UBA1 Screening in Sweet Syndrome With Hematological Neoplasms Reveals a Novel Association Between VEXAS and Chronic Myelomonocytic Leukemia
VV and HJR have contributed equally to this work.
Ethics approval and patients’ consent to participate to the study was approved by The Institutional Review Board of the Cleveland Clinic Foundation. All procedures were carried out in accordance with guidelines set forth by the Declaration of Helsinki.
Written informed consent was obtained from all patients.
Supplemental digital content is available for this article.
VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) is a novel adult-onset nosologic entity of autoinflammatory origin genetically defined by somatic mutations in UBA1 gene mapping on X-chromosome.1 The multifaceted clinical picture of VEXAS encompasses a variety of conditions spanning from hemato-pathological (cytopenias, myeloid vacuoles, plasma cell dyscrasia, and particularly myelodysplastic syndromes [MDS]) to rheumatic disorders (skin manifestations, polychondritis, fever, and pulmonary infiltrates, to name a few).2, 3 The wide medical interest in VEXAS derives from its variegated clinical presentations, juxtaposing for the first time in a prototypic hematoinflammatory disease formerly nosologically unconnected, but often encountered, autoinflammatory, and hematological disorders.
To date, retrospective case series have identified approximately 150 cases gleaning new insights into the VEXAS genophenotypic spectra.4 This syndrome presents during the fifth decade of life predominantly in men. Indeed, in women VEXAS behaves in a pseudoautosomal fashion, as UBA1 escapes the X-inactivation phenomenon.5 Clinically, one of the most common signs of VEXAS is the presence of cutaneous manifestations, reported in up to 90% of cases, with Sweet syndrome or Sweet-like lesions as the most common types of involvement.4, 6 Sweet syndrome is characterized by pyrexia, neutrophilia, painful red nodules or plaques, and neutrophilic infiltrates within the upper dermis, and has been associated with hematological malignancies in up to 85% of paraneoplastic cases.7 Given the intertwined relationship between Sweet syndrome, hematological malignancies, and VEXAS, we aimed to study our internal cohort of cases with both Sweet syndrome and hematological neoplasms to explore the prevalence of VEXAS and raise awareness on the utility of UBA1 screening in such a setting.
To that end, archived data of patients enrolled at the Cleveland Clinic through 2010–2021 were searched for identification of histologically confirmed Sweet syndrome cases, with additional bone marrow (BM) evaluation for hematological neoplasms (further details are provided in Suppl. Appendix). Molecular information from diagnostic gene panels currently used at our center was abstracted when available. DNA sequencing studies for UBA1 gene were carried out as previously described (see also Suppl. Table S1 and Suppl. Appendix).3 The study was approved by the Institutional Review Board of The Cleveland Clinic Foundation in accordance with guidelines set forth by the Declaration of Helsinki.
Overall, 19 patients with clinically and histologically-proven Sweet syndrome and associated BM evaluation were identified from a total of 61 Sweet syndrome cases. Median age at presentation was 65 years (interquartile range, 47–69) with a male-to-female ratio of 0.46 (Table 1). The majority of cases (79%) had co-occurring myeloid neoplasms, while only 4 patients had lymphoid disorders (T-cell large granular lymphocytic leukemia; B-cell acute lymphoblastic leukemia; T-cell lymphoma; IgA kappa multiple myeloma; Figure 1). Sweet syndrome onset was concomitant and constituted the main reason to seek medical attention in the majority of cases, whereas in 2 patients occurred during the disease course (after chemotherapy and during maintenance phase in UPN 15 and 17), and heralded disease relapse in UPN 14. In all cases, skin biopsies showed a strongly CD43-positive infiltrate of neutrophils and histiocytic cells in dermis and subcutis, in perivascular and periadnexal areas, compatible with Sweet syndrome. (Suppl. Figures S1 and S2). Furthermore, skin culture and stains for microorganisms were negative in all patients. Remarkably, Sweet syndrome promptly regressed upon treatment of the underlying hematological disorder and use of appropriate immunosuppressive treatment in UPN 15 and 17.
| UPN | Gender | Age, y | Sweet S. Timing | DNA Available | Vacuoles | UBA1 Sequencing | WHO 2016 Classification | Cytogenetics |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 59 | Concomitant | No | No | - | T-cell lymphoma | - |
| 2 | F | 44 | Concomitant | Yes | No | Negative | CMML-1 | del(13) |
| 3 | M | 57 | Concomitant | Yes | No | Negative | PMF | CK |
| 4 | F | 68 | Concomitant | No | No | - | MDS-U | NK |
| 5 | F | 47 | Concomitant | No | No | - | T-LGLL | - |
| 6 | F | 76 | Concomitant | No | No | - | AML-MRC | CK |
| 7 | F | 73 | Concomitant | Yes | No | Negative | AML with NPM1 Mut | NK |
| 8 | F | 68 | Concomitant | Yes | No | Negative | t-MN | CK |
| 9 | F | 59 | Concomitant | Yes | No | Negative | AML with NPM1 Mut | NK |
| 10 | F | 68 | Concomitant | Yes | No | Negative | t-MN | CK |
| 11 | F | 30 | Concomitant | Yes | No | Negative | AML-NOS | CK |
| 12 | M | 69 | Concomitant | Yes | Yes (Mye) | c.167C>T p.S56F |
CMML-1 | NK |
| 13 | M | 69 | Concomitant | No | No | - | IgA K multiple myeloma | t(11;14) |
| 14 | F | 90 | Post | Yes | No | Negative | AML-NOS | tri(4); t(7;13) |
| 15 | F | 29 | Post | Yes | No | Negative | APL | t(15;17) |
| 16 | F | 65 | Concomitant | Yes | No | Negative | t-MN | CK |
| 17 | F | 5 | Post | No | No | - | B-ALL | t(12;21) |
| 18 | M | 65 | Concomitant | Yes | Yes (Mye/E) | c.122 T>C p.M41 | MDS-MLD | NK |
| 19 | M | 66 | Concomitant | Yes | Yes (Mye/E) | c.122 T>C p.M41 | MDS-SLD | NK |
- AML = acute myeloid leukemia; AML-MRC = acute myeloid leukemia with myelodysplasia-related changes; AML-NOS = AML not otherwise specified; APL = acute promyelocytic leukemia; B-ALL = B-cell acute lymphoblastic leukemia; CK = complex karyotype; CMML-1 = chronic myelomonocytic leukemia- type 1; E = erythroid; F = female; M = male; Mye = myeloid; MDS = myelodysplastic syndrome; MDS-MLD = myelodysplastic syndrome with multilineage dysplasia; MDS-SLD = myelodysplastic syndrome with single lineage dysplasia; MDS-U = myelodysplastic syndrome-unclassifiable; Mut = mutant; NK = normal karyotype; PMF = primary myelofibrosis; T-LGLL = T-cell large granular lymphocytic leukemia; t-MN = therapy-related myeloid neoplasm; WHO = World Health Organization.
Although VEXAS is an X-linked condition, sporadic female patients have been described due to X-skewed clonal hematopoiesis, as well as in acquired and more rarely in constitutional monosomy X cases.8 Therefore, we sequenced 7 female patients with available DNA samples. However, no mutation was found. Overall, we found UBA1 mutations in 3 of 4 tested male patients (n = 2/6 cases had no genomic material), and detected the same variant found in the BM also in the skin sample of one case with available genomic material. Of note is that vacuoles in hematopoietic precursors were present in all VEXAS patients. Nonetheless, when expanding our evaluation also to BM specimens collected at any time point, one additional case from our cohort was found with vacuoles in hematopoietic precursors. In this patient with a therapy-related myeloid neoplasm, the vacuolization phenomenon appeared in a subsequent BM specimen following allogeneic hematopoietic stem cell transplant, probably as a result of the exposure to cytotoxic treatments and in line with our previous report delving into this peculiar feature of VEXAS.3
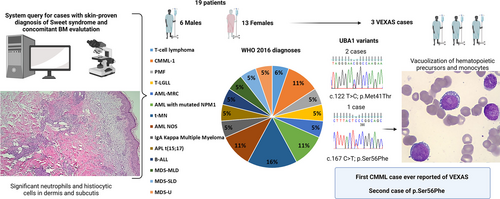
Study design and patients’ features. On the left, schematic representation of the study design illustrating the query of the pathology system to find patients with a co-occurring clinical diagnosis of Sweet syndrome (a skin biopsy from a representative patient is showcased as an example) and a hematologic neoplasm. In the middle section, information on gender and diagnoses according to 2016 WHO of our 19 patients is provided. On the right hand, details on the 3 index cases. In particular, the 2 chromatograms show the forward sequences of 2 patients with the UBA1 canonical hotpot c.122 T>C, p.Met41Thr and one with the rare c.167C>T, p.Ser56Phe. A detail of the bone marrow histopathology of the latter case (UPN12) shows monocytes with intracytoplasmic vacuoles, hallmarks of VEXAS. AML = acute myeloid leukemia; AML-MRC = acute myeloid leukemia with myelodysplasia-related changes; AML-NOS = AML not otherwise specified; APL = acute promyelocytic leukemia; B-ALL = B-cell acute lymphoblastic leukemia; BM = bone marrow; CMML-1 = chronic myelomonocytic leukemia- type 1; MDS myelodysplastic syndrome; MDS-MLD = myelodysplastic syndrome with multilineage dysplasia; MDS-SLD = myelodysplastic syndrome with single lineage dysplasia; MDS-U = myelodysplastic syndrome-unclassifiable; PMF = primary myelofibrosis; T-LGLL = T-cell large granular lymphocytic leukemia; t-MN = therapy-related myeloid neoplasm; VEXAS = vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic; WHO = World Health Organization.
Regarding UBA1 mutations, 2 patients (aged 65 and 66) previously described3 carried the classical c.122 T>C p.Met41Thr with a diagnosis of Sweet syndrome and concomitant low-risk MDS, cytoplasmic vacuoles in myeloid precursors and pronormoblasts, and normal cytogenetics (one had also a DNMT3A p.R882H at a 26% variant allelic frequency; Suppl. Table S2) Both patients had a history of venous thrombosis with one having a previous diagnosis of rheumatoid arthritis. Most interestingly, the third case was a 69-year-old male with the rare c.167C>T p.Ser56Phe mutation, splenomegaly (21 cm) and a medical history remarkable for rheumatoid arthritis, diastolic cardiac failure, Merkel cell and bladder carcinomas. A complete blood count showed 14.4 × 109/L white blood cells with 32% of monocytes, hemoglobin of 9.3 g/dL, and a platelet count of 54 × 109/L. The BM evaluation revealed vacuoles in myeloid precursors and monocytes, 7% blasts, dysplastic features with small hypolobated megakaryocytes and hypogranular granulocytes, and grade-2 reticulin fibrosis compatible with the diagnosis of chronic myelomonocytic leukemia (CMML-1) (Suppl. Figures S3 and S4). Conventional cytogenetics showed normal karyotype while a mutational screening revealed somatic ASXL1 (variant allelic frequency, 14%), FLT3-ITD (21%), and SRSF2 (44%) gene mutations (Suppl. Table S2). Remarkably, we were able to show the presence of the same rare UBA1 p.Ser56Phe mutation in the skin sample of this patient (Suppl. Figure S5). Given the performance status and comorbidities along with personal preferences, the patient was judged not suitable for allogeneic BM transplant and was started on azacitidine considering the prevailing dysplastic over proliferative features of the disease. Unfortunately, after the second cycle and the clinical improvement of Sweet syndrome, the patient suddenly died from bilateral pneumonia.
It is noteworthy that all BM specimens from our study cohort underwent a second evaluation to search for possible VEXAS features. For instance, only an accurate revision of the BM specimen of UPN 12, specifically triggered by the UBA1 testing results, led to the identification of the vacuolization phenomenon in occasional monocytes and blasts. Indeed, VEXAS cases with subtle BM changes and rare vacuoles have also been described, as this feature of the syndrome is typical but not pathognomonic.9 To cite an example of a BM morphologic feature with similar characteristics to vacuoles in VEXAS, Auer rods make the best comparison. In fact, just few or rare cells showing this particular aspect are sufficient to drive the pathologist's attention towards particular disease spectra but not unique diagnostic entities (eg, various high-grade myeloid neoplasms for Auer rods or peculiar benign and malignant conditions for vacuoles), and without the strict requisite of an actual threshold.10 A recent study showed that while the sole presence of vacuoles in myeloid precursors was not specific to VEXAS, the presence of ≥10% of neutrophil precursors with >1 vacuole had excellent sensitivity and specificity in recognizing UBA1 mutant cases.11 However, no specific consensus currently exists on this topic. The difficulty in studying retrospectively the vacuolization phenomenon relying only on medical chart review explains the discrepancy between the present and our previous study,3 where at least 2 of the currently presented patients were not recognized.
Since its discovery about 1 year ago,1 various groups have expanded as to the genetics, the pleomorphic clinical phenotypes, and the possible treatment options of VEXAS in retrospective case series identified sequencing patients samples according to clinical suspicion. In addition to the most common p.Met41, other 4 pathogenic mutations have been described: a group of splice acceptor site variants (c.118-1G>C, c.118-2A>C and c.119-1G>C) and a p.Ser56Phe variant identified in one previous case.4, 12, 13 In contrast to the classical p.Met41 and the splice site variants, both resulting in reduced cytoplasmic UBA1 levels (quantitative effect), the p.Ser56Phe mutation affects the catalytic activity of UBA1 leading to a temperature-dependent reduction of ubiquitination (qualitative effect).13 Of note is that the only one patient previously described with the latter mutation also had Sweet syndrome, low-risk MDS, and a major rheumatologic disorder (polymyalgia rheumatica) but no mention of BM vacuoles, possibly suggesting that the different pathogenic mechanism of this variant may result in a deviation from some of the VEXAS-defining features (eg, minimal/occasional presence of vacuoles).13
Finally, while systemic inflammatory and autoimmune disease have been traditionally reported to be associated with both MDS and CMML patients, only MDS has been so far reported with VEXAS,1, 14 even when specifically studying cohorts including patients with co-occurrence of CMML and autoinflammatory disorders.15, 16 Therefore, we here present not only the second case ever described with the p.Ser56Phe rare variant but also the first VEXAS patient with a diagnosis of CMML-1. Furthermore, while we acknowledge that genetic testing was not available for all cases, our study demonstrates the co-existence of VEXAS in patients with hematological neoplasms and Sweet syndrome, emphasizing the need for considering this syndrome in the differential diagnoses of male patients presenting with such a combination of clinical features.
Future studies on selected patient populations characterized by VEXAS-related features may be helpful to shed light onto its clinical manifestations, expanding the existing knowledge of the disease.
ACKNOWLEDGMENTS
We thank grants: R35HL135795 (to JPM), AA&MDSIF (to VV and SP), Vera and Joseph Dresner Foundation–MDS (to VV), Italian Society of Hematology (to SP), Tito Bastianello Young Investigator Award (to SP and CG), AIRC 5 × 1000 call “Metastatic disease: the key unmet need in oncology to MYNERVA” project, #21267 (MYeloidNEoplasms Research Venture AIRC), and MIUR grant N. 2017WXR7ZT (both to MTV).
AUTHOR CONTRIBUTIONS
CG, VV, and HJR generated and conceived the study design, figures, tables, and manuscript; IP and VV performed sequencing data. HJR and PM collected and analyzed pathology data and provided detailed morphologic information. SP, MTV, and JPM provided clinical expert input on the manuscript. All authors participated in data interpretation and critical review of the final paper and submission.
DISCLOSURES
The authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
This work was supported by a grant from the Edward P. Evans Foundation (to C.G.).