Influence of Telomere Length on the Achievement of Deep Molecular Response With Imatinib in Chronic Myeloid Leukemia Patients
Data that support the findings of this study are available from the corresponding author upon request.
Supplemental digital content is available for this article.
Abstract
Tyrosine kinase inhibitors have dramatically changed the outcome of chronic myeloid leukemia (CML), and nowadays, one of the main treatment goals is the achievement of deep molecular responses (DMRs), which can eventually lead to therapy discontinuation approaches. Few biological factors at diagnosis have been associated with this level of response. Telomere length (TL) in peripheral blood cells of patients with CML has been related to disease stage, response to therapy and disease progression, but little is known about its role on DMR. In this study, we analyzed if age-adjusted TL (referred as “delta-TL”) at diagnosis of chronic phase (CP)-CML might correlate with the achievement of DMR under first-line imatinib treatment. TL from 96 CP-CML patients had been retrospectively analyzed at diagnosis by monochrome multiplex quantitative PCR. We observed that patients with longer age-adjusted telomeres at diagnosis had higher probabilities to achieve DMR with imatinib than those with shortened telomeres (P = 0.035 when delta-TL was studied as a continuous variable and P = 0.047 when categorized by the median). Moreover, patients carrying long telomeres also achieved major molecular response significantly earlier (P = 0.012). This study provides proof of concept that TL has a role in CML biology and when measured at diagnosis of CP-CML could help to identify patients likely to achieve DMR to first-line imatinib treatment.
Introduction
Chronic myeloid leukemia (CML) is a clonal hematopoietic stem cell disease caused by the acquisition of a reciprocal translocation between chromosomes 9 and 22, producing the so-called Philadelphia chromosome. This translocation encodes for the constitutively active tyrosine kinase BCR-ABL1, which promotes exacerbated myeloproliferation, resistance to apoptosis and survival advantage.1
Imatinib, the first tyrosine kinase inhibitor (TKI) developed, is rather selective for the BCR-ABL1 oncoprotein and has dramatically improved the outcome of CML patients. This drug is capable of inducing complete cytogenetic response in the majority of chronic phase CML (CP-CML) patients, and almost all these patients normally achieve a major molecular response (MMR), defined as BCR-ABL1 ≤0.1% on the international scale (IS). However, with imatinib, only 30%–40% of patients achieve a deep molecular response (DMR) defined as either MR4.0, BCR-ABL1IS ≤0.01% or MR4.5, BCR-ABL1IS ≤0.0032%),2 while with the introduction of second and third generation TKI at first line, such as nilotinib, dasatinib, and bosutinib, higher rates as well as earlier achievement of DMR have been observed.3-5 Recent clinical trial data have demonstrated that TKI therapy can be safely discontinued in patients with sustained DMR (sDMR), leading to a successful long-term treatment-free remission (TFR) in 40%–50% of patients.6, 7 In clinical practice, the TFR rate is slightly higher (~65%), likely due to longer duration of both TKI exposure and sDMR, compared to clinical trials.8, 9 Thus, the achievement of DMR has recently become one of the most important treatment goals in CML.
Several clinical scores, such as Sokal, European Treatment and Outcomes Study (EUTOS), Hasford and EUTOS long-term survival (ELTS),10-13 are used to predict the outcomes of patients diagnosed with CP-CML, although there are no clear recommendations regarding the most suitable TKI, especially in low-risk disease.14 Currently, the best way to adjust TKI treatment is by assessing response at different milestones. One of these is the “early molecular response”; a BCR-ABL1/ABL1IS ratio of >10% at 3 months from TKI initiation has been related to a worse response later on and even worse overall survival.15 Another example is the “halving time,” which evaluates the kinetics of decline of BCR-ABL1 transcripts during the first 3 months of treatment.16 Moreover, Sasaki et al17 evaluated the best-fit average and minimum acceptable BCR-ABL1 levels within 1 year (from 3 to 12 mo) of treatment to predict the achievement of sDMR at any point. However, all this prognostic information is obtained while the patient is already under treatment (ie, “semiprospectively”) and not at diagnosis, thus clinical decisions must be suddenly changed once the TKI has been chosen and milestones established are not met. Knowing the probabilities to achieve DMR with imatinib at diagnosis using molecular markers could improve patient care and help to choose the best TKI (imatinib versus a more potent second or third generation TKI) when TFR is the main treatment goal.
Several studies have defined molecular markers that can predict response to imatinib at diagnosis, such as OCT-1, ABCB1, and PTCH1 expression levels or polymorphic variants,18-20 as well as a model based on a multigene expression signature, which is able to identify patients who are at very high risk of early molecular response failure.21 However, none of these markers has been correlated with DMR. So far, the only biomarker that has been related to higher rates of DMR achievement in some but not all studies is the e14a2 transcript type.22-24
Telomeres represent a promising predictive molecular marker in CML. Telomeres are repeat DNA sequences (TTAGGG) located at the end of the chromosomes. In somatic cells, telomeres shorten with each cell division, reflecting the replicative history of a cell, and can eventually lead to genetic instability and cellular senescence. In CML, increased cellular turnover of BCR-ABL1-positive stem and progenitor cells leads to significantly shortened telomeres in peripheral blood (PB) myeloid cells.25 Mechanistically, an inflammatory phenotype called “telomere-associated secretory phenotype” has been suggested to contribute, via secretion of chemokines and interleukins, to BCR-ABL1-mediated growth and thus CML onset.26
Accelerated telomere shortening in CML has previously been correlated with disease stage, clinical risk scores at diagnosis, cytogenetic remission status as well as progression to accelerated phase or blast crisis.27, 28 Moreover, telomere length (TL) at diagnosis of CML has been associated with MMR at 12 and 18 months of first-line nilotinib treatment.29
Altogether, these data provide further evidence for the crucial role of telomere biology in CML, especially in terms of response and disease progression. To our knowledge, no study has explored the association between TL at diagnosis and the achievement of DMR to first-line imatinib treatment.
Therefore, in this study, we retrospectively analyzed mean age-adjusted TL (referred as delta-TL) in CML patients at diagnosis. We evaluated whether delta-TL would be useful to identify individuals likely to achieve sDMR with first-line imatinib treatment. Additionally, we aimed to (1) evaluate the association between delta-TL at diagnosis and risk scores as well as BCR-ABL1-p210 transcript type; (2) analyze if delta-TL at diagnosis has an impact on the achievement of optimal MR according to European LeukemiaNet (ELN) recommendations30; and (3) determine if there are differences in terms of delta-TL at diagnosis between patients that do achieve MMR but may or not achieve MR4.0.
Material and methods
Patients, samples, and study design
A total of 96 adult patients with CP-CML were enrolled in this study. Patients were consecutively diagnosed in ICO-Hospital Germans Trias i Pujol (n = 36), ICO-Hospital Duran i Reynals (n = 38), and ICO-Hospital Josep Trueta (n = 22), between 2005 and 2016, according to the 2008 World Health Organization (WHO) classification.31 Retrospective DNA samples from PB and bone marrow (BM) at diagnosis were collected from all cases.
Study approval was obtained from Institut Català d'Oncología-Hospital Germans Trias i Pujol Ethics Committee (Ref. PI-17-261), and informed consent was provided by all the patients. The study was undertaken in accordance with the Declaration of Helsinki.
All patients were selected according to the following criteria: (1) first-line treatment with imatinib, 400 mg orally once a day; (2) a minimum of 1 year of follow-up; (3) absence of toxicity or intolerance to imatinib that required a change of TKI treatment or a dose reduction; and (4) the absence of mutations in ABL1 gene or additional cytogenetic abnormalities.
In this study, TL of CML patients was adjusted for age using PB samples from 107 healthy subjects.
DNA extraction
DNA from PB (whole blood) samples at CML diagnosis was used, because positive correlation between TL measured with this type of sample has been observed.32 DNA from PB samples was not available in 29 patients and in those cases DNA from BM samples was used, as at diagnosis no TL differences between these 2 different samples have been shown.33
DNA was extracted automatically using QIAcube (Qiagen, Germany) with the QIAamp Blood Mini Kit (Qiagen). DNA concentration and quality status were assessed with a NanoDrop spectrometer.
Fresh PB samples from 107 age-matched healthy subjects (age range 16–84 y) were used for TL age-adjustment, applying linear regression analysis, and DNA was extracted as described above.
We used a PB sample from a healthy donor to generate a standard curve for the monochrome multiplex (MM)-qPCR method. DNA was extracted manually using the Gentra Puregen Blood Kit (Qiagen) as it allows higher DNA concentrations to be obtained.
Definition of molecular response
qPCR was used for measuring BCR-ABL1 transcripts, as described previously.34 ABL1 was used as the control gene, and the results were reported as %BCR-ABL1/ABL1IS. Molecular monitoring was performed at diagnosis (baseline) and every 3–6 months of imatinib treatment thereafter.
The 2020 ELN recommendations30 were considered to assess MR to first-line imatinib treatment, that is: BCR-ABL1/ABLIS ≤10% at 3 months, <1% at 6 months and ≤0.1% (MMR) at 12 months and later. Moreover, the primary study end-point included the achievement of sDMR, defined as MR4.0 and/or MR4.5, confirmed on 2 or more consecutive determinations
Telomere length measurements by MM-qPCR
TL was analyzed by MM-qPCR, as previously described by Cawthon,35 using a CFX384 Touch Real-Time PCR Detection System (Bio-Rad, Spain), and followed previously used protocols.36, 37 All samples, standards, and controls were run in triplicate, and the median value was used for the analyses. TL was calculated as a ratio between fluorescence detected from telomere repeat copy number (T) and a single copy reference sequence (S) from the human beta globin gene. Primer pairs used for telomere (T) amplification were telg 5′-ACACTAAGGTTTGGGTTTG GGTTTGGGTTTGGGTTAGTGT-3′ and telc 5′-TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA-3′, and signal acquisition was at 74°C. Human beta-globin was used as single copy reference gene (S) using the primers hbgu 5′-CGGCGGCGGGCGGCGCGGGCTGGG CGGCTTCATCCACGTTCACCTTG-3′ and hbgd 5′-GCCCGGCCCGCCGC GCCCGTCCCGCCGGAG GAGAAGTCTGCCGTT-3′, and signal acquisition was at 88°C. CFX Manager Software (Bio-Rad) was used to analyze the raw data and generate 2 standard curves (one for each signal acquisition temperature; for the telomere signal and for the single copy gene signal). Samples values were normalized and the telomere/single copy gene ratio (T/S ratio) was calculated for each DNA sample.
The age-adjusted T/S ratio (or age-adjusted TL), hereafter referred to as delta-TL, was calculated using data from 107 healthy subjects. Delta-TL represents the difference between the CML patient's T/S ratio, calculated by MM-qPCR, and the corresponding TL expected according to age (calculated also by MM-qPCR using data from healthy subjects), with higher delta-TL values meaning shorter telomeres. All statistical analyses were performed using the delta-TL value for each patient.
In this study, the average intra-assay variability for all samples (n = 203, control and CML samples included) was 8%. To monitor plate-to-plate variation, 4 reference samples (2 with known long telomeres and 2 with known short telomeres) were included in each run and the resulting average inter-assay variability was 14%.
Statistical analysis
The study group characteristics were described as frequency and percentage for categorical variables and median and range for quantitative variables. Comparisons of continuous variables between groups were made using the median test, while categorical variables were compared using the χ2 test or Fisher exact test, if necessary.
Due to the retrospective nature of this study including real-world data and in line with previous studies,38, 39 competing risk analysis was used for all the time-dependent variables. The achievement of MMR and/or DMR to imatinib was considered as the main event and the associated time was considered from imatinib start to documented MR (MMR, MR4.0 or MR4.5, depending on the analysis). Patients who switched TKI without achieving documented MR or those who did not change TKI but died without MR response, were considered as competitive events; time from imatinib start to TKI change or death was considered for these competitive events. Alive patients without TKI change and without documented MR were considered as censures, and the associated time in these cases was the time from imatinib start to last follow-up. Cumulative incidence curves of MR were plotted and a multivariate analysis for MR4.0 was performed by the Fine and Gray model.
Two-sided P values <0.05 were considered statistically significant. The statistical packages SPSS version 24.0 (SPSS Inc., Chicago, IL) and R v4.0.1 software were used for all the analyses and the creation of graphics. GraphPad Prism 5 software was also used for the creation of graphics and figures.
Results
Clinical and biological characteristics of the patients of the series
The series included a total of 96 CP-CML patients: 56 (58%) males and 40 (42%) females, with a median age at diagnosis of 49 years (range 24–80 y). According to the Sokal score, 46 (50%) patients were of low risk, 30 (32%) were of intermediate risk, and 17 (18%) were of high risk. According to the EUTOS score, 77 (88%) and 11 (12%) were of low and high risk, respectively. Finally, according to the ELTS score, 50 (59%) patients were of low risk, 24 (29%) were of intermediate risk, and 10 (12%) were of high risk. Thirty-one (39%) patients had the BCR-ABL1-p210 e13a2 transcript type, whereas 49 (61%) had the e14a2 transcript. The median follow-up of alive patients was 7.3 years (range, 1.3, 14.6 y). The median length of front-line imatinib treatment was 3.4 years (range, 0.3, 14.4 y). In total, 10 patients (10.4%) were exitus (Table 1).
| Total | Delta-TL<0.4 | Delta-TL≥0.4 | ||
|---|---|---|---|---|
| Clinical Characteristics | n = 96 | n = 47 | n = 49 | P |
| Median age at diagnosis, y (range) | 49 (24–80) | 59 (30–80) | 43 (24–77) | ** |
| Sex | ns | |||
| Female, n (%) | 40 (42) | 20 (43) | 29 (59) | |
| Male, n (%) | 56 (58) | 27 (57) | 20 (41) | |
| Sokal risk score, n (%) | ns | |||
| Low | 46 (50) | 22 (50) | 24 (49) | |
| Intermediate | 30 (32) | 16 (36) | 14 (29) | |
| High | 17 (18) | 6 (14) | 11 (22) | |
| EUTOS score, n (%) | * | |||
| Low | 77 (88) | 40 (95) | 37 (80) | |
| High | 11 (12) | 2 (5) | 9 (20) | |
| ELTS score, n (%) | ns | |||
| Low | 50 (59) | 26 (63) | 24 (56) | |
| Intermediate | 24 (29) | 11 (27) | 13 (30) | |
| High | 10 (12) | 4 (10) | 6 (14) | |
| p210 isoform, n (%) | ns | |||
| e13a2 | 31 (39) | 14 (36) | 17 (42) | |
| e14a2 | 49 (61) | 25 (64) | 24 (58) | |
| Imatinib treatment duration, y (range) | 3.4 (0.3–14.4) | 3.2 (0.3–14.4) | 3.8 (0.3–14) | ns |
| Median follow-up, y (range) | 7.3 (1.3–14.6) | 6.7 (1.5–14.4) | 7.1 (1.3–14.6) | ns |
| Exitus, n (%) | 10 (10.4) | 4 (8.5) | 6 (12.2) | ns |
- ELTS = EUTOS long-term survival; EUTOS = European treatment and outcomes study; ns = not significant.
- *P < 0.05; **P < 0.001.
Regarding TL, healthy controls showed the expected decline with increasing age (R2 = 0.1693), a characteristic that was not detectable in CML patients (R2 = 0.0046) (Figure 1).
We also tested the correlation of both sample sources used in our CML cohort and found no differences in the T/S ratio between PB and BM (median T/S ratio [95% confidence interval (CI)]: 0.60 (0.59, 0.71) versus 0.55 (0.51, 0.66), respectively, P = 0.28, Supplemental Digital Figure S1; http://links.lww.com/HS/A207).
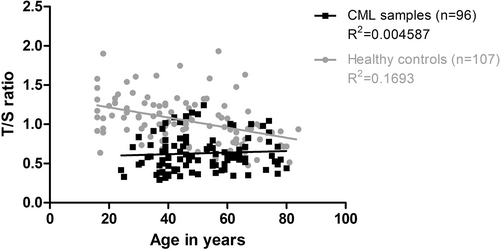
Telomere length of chronic myeloid leukemia patients and healthy controls in relation to age. Relative TL expressed as T/S ratio plotted against age; healthy donors (gray circles) show the expected decline in TL with increasing age (r2 = 0.1693), whereas CML patients (black squares) runs nearly horizontally (r2 = 0.0046). CML = chronic myeloid leukemia; T/S = telomere/single copy gene ratio; TL = telomere length.
Correlation between delta-TL at diagnosis and the achievement of deep molecular response to first-line imatinib treatment
We examined the correlation between achieving or not MR4.0 and MR4.5 and the delta-TL at diagnosis as a continuous variable and observed that lower delta-TL at diagnosis was significantly associated with the achievement of stable MR4.0 and MR4.5 (hazard ratio [HR] [95% CI]: 0.3 (0.1, 0.9), P = 0.035 and HR [95% CI]: 0.22 (0.1, 0.66), P = 0.007, respectively).
Then, we categorized delta-TL into quartiles (Q1: 0.06 ± 0.15; Q2: 0.34 ± 0.03; Q3: 0.48 ± 0.05; Q4: 0.67 ± 0.08) and studied the cumulative incidence of achieving MR4.0 at any time; patients carrying longer telomeres at diagnosis (Q1) presented the highest rate of MR4.0 achievement, followed by patients allocated in Q2. Due to similar cumulative incidence of MR4.0 curves between Q1 and Q2, and also between Q3 and Q4, we considered median delta-TL value (ie, delta-TL = 0.4) as the cutoff for this variable. Descriptively, we found a dose-dependent correlation, meaning that the longer the telomeres at diagnosis, the higher the probabilities of achieving DMR (Supplemental Digital Figure S2; http://links.lww.com/HS/A208).
Therefore, when patients were stratified according to median delta-TL value, the cumulative incidence (95% CI) of MR4.0 at any time between the 2 groups was 63% (45%, 76%) for delta-TL <0.4 and 46% (31%, 60%) for delta-TL ≥0.4 (P = 0.047). Median cumulative incidence of MR4.0 in delta-TL <0.4 group was at 27 months from imatinib start, while this point was not reached in delta-TL ≥0.4 group, since cumulative incidence for MR4.0 in this subpopulation was <50% (Figure 2A).
Descriptively, 29 (62%) of 47 patients in delta-TL <0.4 group and 22 (45%) of 49 of delta-TL ≥0.4 group achieved MR4.0 with a median (range) time of 14 months (6.2, 71.4) and 17 months (5.5, 100.7), respectively.
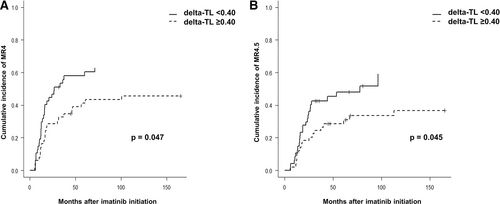
Cumulative incidence (95% CI) of MR4.0 and MR4.5 according to delta-TL categorized by the median. (A) Cumulative incidence of MR4.0. (B) Cumulative incidence of MR4.5. CI = confidence interval; TL = telomere length.
Regarding MR4.5, the cumulative incidence (95% CI) was 59% (36%, 76%) for the delta-TL <0.4 group and 37% (22%, 52%) for the delta-TL ≥0.4 group (P = 0.045), and for patients allocated in the delta-TL <0.4 group, median cumulative incidence of MR4.5 was 77 months from imatinib start, while it was not reached in the delta-TL ≥0.4 group (Figure 2B).
Moreover, cumulative incidences (95% CI) of MR4.0 were studied establishing a cutoff at 4 years, being 58% (44%, 70%) for the delta-TL <0.4 group and 39% (26%, 52%) for delta-TL ≥0.4 (P = 0.049). Cumulative incidences of MR4.5 at 4 years were 45% (30%, 59%) for the delta-TL <0.4 group and 29% (17%, 42%) for delta-TL ≥0.4 (P = 0.08) (Supplemental Digital Figure S3; http://links.lww.com/HS/A209).
We also explored if there were differences between CML patients that did achieve MMR (n = 64) but did or did not achieve MR4.0 at any time. Analyzing the incidence of MR4.0 in this subpopulation (only patients that achieved MMR), we observed an earlier achievement of MR4.0 in the delta-TL <0.4 group, but differences were not statistically significant (median in months [95% CI]: 16.3 (12.4, 33.4) in delta-TL <0.4 versus 31.8 (16.2, 100.7) in delta-TL ≥0.4, P = 0.144). In this subgroup of patients, cumulative incidence (95% CI) of MR4.0 was 84% (65%, 94%) for delta-TL <0.4 and 75% (54%, 87%) for delta-TL ≥0.4 (Supplemental Digital Figure S4; http://links.lww.com/HS/A210).
Correlation between delta-TL at diagnosis, risk scores, and BCR-ABL1-p210 transcript type
Associations between delta-TL and prognostic variables of clinical relevance (Sokal, Eutos, ELTS, and BCR-ABL1-p210 transcript type) were examined to find any possible correlation with TL at diagnosis. We did not observe any association with the prognostic risk scores (Sokal, EUTOS, and ELTS) nor with transcript type when delta-TL was studied as a continuous variable. However, when delta-TL was categorized by the median, we found a statistically significant correlation with the EUTOS score (P = 0.036), given that only two patients out of 42 (5%) with delta-TL <0.4 were classified within the EUTOS high-risk group (Table 1).
Multivariate analysis for MR4.0 achievement
Moreover, we performed a multivariate analysis to study the role of delta-TL as an independent predictor of MR4.0. Variables significantly associated with MR4.0 in univariate analysis were selected; delta-TL and ELTS score showed statistical significant differences in MR4.0 between their categories, reaching higher cumulative incidence of MR4.0 in delta-TL <0.40 and low risk ELTS score (cumulative incidence of MR4.0 [95% CI]: 63% [45%, 76%] for delta-TL <0.4 versus 46% [31%, 60%] for delta-TL ≥0.4, P = 0.047, and 58% [42%, 71%] for low risk ELTS versus 38% [21%, 55%] for intermediate-high risk ELTS, P = 0.041). However, in the multivariate analysis, delta-TL lost its significance (HR [95% CI]: 1.7 (0.92, 3.22), P = 0.089), whereas ELTS score retained its significance as an independent predictor of MR4.0 (HR [95% CI]: 1.9 (1.02, 3.73), P = 0.045) (Table 2).
| Univariate Analysis | |||
|---|---|---|---|
| Variable | Cumulative Incidence MR4.0 (95% CI) | P | |
| delta-TL | < 0.40 (n = 47) | 63% (45%, 76%) | 0.047 |
| ≥ 0.40 (n = 49) | 46% (31%, 60%) | ||
| Sokala | Low (n = 46) | 50% (37%, 61%) | 0.312 |
| Int-High (n = 47) | 26% (14%, 39%) | ||
| EUTOS | Low (n = 77) | 56% (43%, 66%) | 0.414 |
| High (n = 11) | 36% (8%, 66%) | ||
| ELTSa | Low (n = 50) | 58% (42%, 71%) | 0.041 |
| Int-High (n = 34) | 38% (21%, 55%) | ||
| Isoform | e13a2 (n = 31) | 42% (23%, 60%) | 0.151 |
| e14a2 (n = 49) | 55% (40%, 68%) | ||
| Multivariate Analysis | |||
| Variable | Reference Category | HR (95% CI) | P |
| delta-TL | ≥ 0.40 | 1.7 (0.92, 3.22) | 0.089 |
| ELTS | Int-High | 1.9 (1.02, 3.73) | 0.045 |
- aSokal and ELTS scores have been regrouped into 2 categories (low versus intermediate-high) in order to balance the number of patients between categories.
- CI = confidence interval; ELTS = EUTOS long-term survival; EUTOS = European treatment and outcomes study; HR = hazard ratio.
- Bold values mean they are statistically significant (P < 0.05).
Correlation between delta-TL at diagnosis and molecular response according to the 2020 ELN recommendations
Finally, we studied the relation between TL at diagnosis and optimal molecular response to treatment according to the 2020 ELN recommendations.30
When analyzing delta-TL as a continuous variable, we did not find any association between delta-TL and the cutoffs established by ELN for BCR-ABL1/ABL1(IS) ratio at 3, 6, nor 12 months after initiation of imatinib treatment (Supplemental Digital Table S1; http://links.lww.com/HS/A206). However, we observed a trend toward a correlation between delta-TL and the achievement of MMR at 18 months (P = 0.075), indicating that patients with longer telomeres at diagnosis could have a higher probability of achieving MMR at 18 months with imatinib than patients with shorter telomeres. This association was confirmed when delta-TL was categorized by the median, with 78% and 56% of patients with delta-TL <0.4 and delta-TL ≥0.4, respectively, achieving MMR at 18 months (P = 0.049) (Supplemental Digital Table S1; http://links.lww.com/HS/A206). Finally, cumulative incidence analysis (95% CI) of MMR at 24 months from imatinib start also supported this finding, being 68% (55%, 78%) for the delta-TL <0.4 group and 43% (30%, 56%) for delta-TL ≥0.4 (P = 0.012) (Figure 3).
Discussion
Treatment with TKIs has dramatically improved CML outcomes up to the point of introducing the concept of TFR in optimal responders. However, there is still a lack of predictive molecular markers that could prospectively identify patients with a high likelihood of achieving a DMR with a particular TKI, thus allowing them to follow a strategy aimed at the achievement of a TFR right from treatment initiation. In this study, we retrospectively evaluate if TL assessment by MM-qPCR at diagnosis of 96 CP-CML patients could be useful to identify those patients. In line with previous models, our results now show clinically that patients with shortened telomeres at diagnosis (ie, a high delta-TL value) are likely to fail in achieving DMR (both MR4.0 and MR4.5) compared to those with longer telomeres (Figure 2).
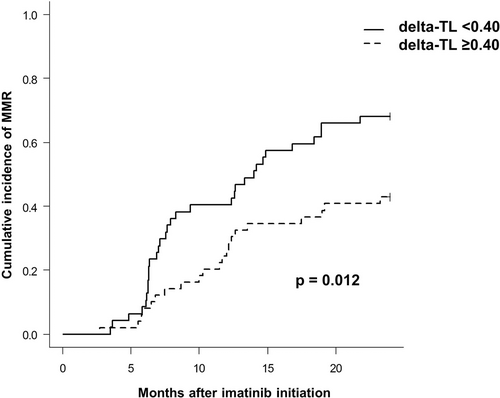
Cumulative incidence (95% CI) of MMR at 24 months from imatinib start. CI = confidence interval
To our knowledge, the only study that has related TL with molecular response to TKI showed that TL at diagnosis of CML was associated with MMR achievement at 12 and 18 months under first-line nilotinib treatment.29 Even though it was not the main endpoint of our study, we also observed statistically significant differences in cumulative incidence of MMR between delta-TL <0.4 and delta-TL ≥0.4 groups, which started to be evident after 6 months of imatinib onset (Figure 3). Moreover, in our study we showed that longer telomeres (delta-TL < 0.4) were positively correlated with the primary study endpoint, that is, a superior molecular response defined as MR4.0 and MR4.5. In this case, the largest differences between the 2 categorical groups (delta-TL <0.4 versus delta-TL ≥0.4) in terms of DMR were mostly observed after 2 years of treatment (Figure 2), in line with previous clinical trial data, in which cumulative rates of MR4.0 with imatinib were reported to be around 60% after 10-year follow-up.2-5
Caocci et al40 found a significant correlation between short age-adjusted TL and higher rates of TFR. However, in this study TL was measured using PB samples obtained during the discontinuation phase (off TKI), when residual disease is minimal and often undetectable. In this scenario, in which normal hematopoiesis predominates, this correlation may not be explained by CML itself.
We also confirmed that TL is significantly shortened in CML PB and BM leukemic cells at diagnosis compared to healthy donors, and it is no longer age-dependent, consistent with previous literature.28 Bouillon et al41 recently showed that the degree of TL shortening measured in the CD34+CD38− leukemic stem cell compartment of CML can discriminate between early from late CP-CML and it can be directly related to the size of the leukemic clone. In line with this study, we observed that, even though TL in CML patients was generally shortened compared to age-matched healthy controls, there was an important variability in TL between patients at diagnosis, possibly indicating distinct CP-CML disease substages. Of clinical relevance, we believe that TL measured in PB or BM could be used not only as a biomarker for TKI molecular response but also to estimate the duration of CP-CML from its onset.
In our study, we did not find any correlation between prognostic risk scores and delta-TL at diagnosis. Only when delta-TL was categorized by the median, we did find a significant association with EUTOS score (P = 0.036), but this finding would need to be validated. Moreover, the BCR-ABL1-p210 transcript type did not show any association with TL, suggesting that the type of transcript may not have any impact on the leukemic cell division rate.
Univariate analysis showed statistically significant cumulative incidence of MR4.0 both for delta-TL and ELTS score. However, only ELTS retained significance in multivariate analysis, although delta-TL showed a trend toward higher incidence of MR4.0 in low delta-TL patients.
We also evaluated the impact of delta-TL at diagnosis on achieving the different degrees of MR at 3, 6, and 12 months, according to the 2020 ELN recommendations, but no correlation was observed. Only at 18 months of imatinib treatment, patients with delta-TL <0.4 at diagnosis significantly achieved MMR compared to those with delta-TL ≥0.4. In line with this observation, Wenn et al29 described a correlation between longer TL at diagnosis of CML and achievement of MMR at 12 and 18 months with first-line nilotinib treatment, but they could not confirm this association at 3 or 6 months.
As far as we know, the only study that has reported the use of a biomarker to predict DMR to imatinib therapy is the one recently published by Park et al.42 In this study, they described a polymorphism in HMGCLL1 gene that predicts intrinsic sensitivity to imatinib therapy which may be used to identify those patients at risk of not achieving DMR. Moreover, they postulated that HMGCLL1 blockade could potentially sensitize leukemic stem cells to TKI therapy, although no preclinical data are available. Despite being a promising marker, it needs further validation. In contrast, telomere biology has been widely related to CML pathology and has already been validated as a useful marker in several studies.25-29
Despite our good results, we are aware that quantitative fluorescence in situ and flow cytometry (flow-FISH) based methodologies have shown to be more accurate than MM-qPCR for TL measurement in vivo,36, 43 but flow-FISH requires fresh viable PB or BM samples and this material was not available because of the retrospective nature of our study. Validation of our results in large prospective studies using flow-FISH would be of special interest.
In summary, telomere length measured by MM-qPCR in either PB or BM samples at diagnosis of CML identified a previously unrecognized patient subgroup likely to achieve both MMR and DMR with front-line imatinib treatment. We believe that telomere length measurement could complement other clinical prognostic scores at diagnosis (such as ELTS), providing additional information about the probability of DMR achievement. This molecular marker may be useful to guide the choice of TKI at diagnosis when DMR achievement is the objective as a road to treatment discontinuation, especially in young patients. Unlike other milestones (such as early molecular response or halving time), this marker could predict long-term outcomes (in terms of MR) before any TKI is started.
Acknowledgments
The authors thank Josep-Maria Ribera and Diana Dominguez for their help.
Disclosures
B.X. and L.Z. received research funding from Novartis, Celgene, and Incyte (not related to this study). All the other authors have no conflicts of interest to disclose.
Sources of funding
This work was in part supported by a grant from Instituto de Salud Carlos III through the project “PI16/01200” (Co-funded by European Regional Development Fund/European Social Fund “A way to make Europe”/”Investing in your future”) as well as by the grant “Bolsa de ayuda económica para la innovación científica” and the grant “Becas de Investigación” both from Fundación Española de Hematología y Hemoterapia (FEHH).