Reticulocytosis As a Whistleblower: A Rare Case of Acquired Elliptocytosis in a Myelodysplastic Syndrome Patient With Trisomy 8
MHS and JZ contributed equally to this work.
Elliptocytosis is a corpuscular erythrocyte abnormality characterized by the presence of oval or elliptical erythrocytes on the blood smear. In most cases, it is an inherited condition, but a few cases of acquired elliptocytosis (AE) have been described in myeloid malignancies, most often in myelodysplastic syndrome (MDS) associated with del(20q).1 This report describes the first case of AE in a patient presenting with regenerative anemia and dysgranulopoiesis leading to the diagnosis of MDS with isolated trisomy 8. AE was associated with rapid transformation in our patient, which will be discussed in light of the data from the literature.
A 69-year-old man was referred to the internal medicine department of our institution for exploration of a bicytopenia with a subacute anemia (9.3 g/dL), which was normocytic (96 fL) and highly regenerative (reticulocytosis 235 G/L), and a mild thrombocytopenia (115 G/L). White blood cell count was within normal ranges (7.4 G/L) with an absolute neutrophil count of 4.39 G/L. He had presented progressive asthenia for 3 months with marked weight loss in the last 3 weeks despite a preserved appetite, dyspnea, and chest pain upon exercise, preventing him from performing his daily activities. This patient had a history of hypertension and hereditary hemochromatosis treated by phlebotomy. Physical examination revealed a slight splenomegaly and no externalized bleeding.
Biological exploration showed hemolysis with a low haptoglobin (0.34 g/L), a moderately increased total bilirubin (40 μmol/L), and normal lactate dehydrogenase (226 International Units/L). Peripheral blood smear showed marked poikilocytosis (numerous ovalocytes, few elliptocytes, acanthocytes, fragmented red cells, and teardrop cells) without schistocytes (Figure 1A). Direct antiglobulin test, search for pyruvate kinase and glucose-6-phosphate dehydrogenase deficiency, and paroxysmal nocturnal hemoglobinuria clone were negative and no medication was recently introduced. Digestive endoscopy and colonoscopy revealed no abnormal findings.
As blood smear examination also revealed dysplasia (hyposegmentation and degranulation of neutrophils) (Figure 1B), a bone marrow aspiration was performed and showed a medium richness with signs of multilineage dysplasia, no excess blast, confirming the diagnosis of MDS with multilineage dysplasia.2 Conventional cytogenetic analysis showed an abnormal karyotype with presence of trisomy 8 (47,XY,+8 [8]/46,XY [12]), stratifying this patient as having an intermediate prognosis (Revised International Prognostic Scoring System).3 Molecular analyses revealed 5 mutations in TET2 (n = 2, variant allele frequencies [VAFs] = 37% and 17%, respectively), ASXL1 (VAF = 30%), EZH2 (VAF = 34%), and STAG2 (VAF = 41%) genes.4 An unusual very high reticulocyte count gradually increasing to 340 G/L and ultimately to 581 G/L, along with increasing bilirubin and lactate dehydrogenase to 56 μmol/L and 356 U/L, respectively, a strong decrease in haptoglobin to 0.08 g/L (Figure 2, A and B), and major poikilocytosis in this MDS context led to further explorations. An osmotic gradient ektacytometry test was performed, whose profile was suggestive of an atypical elliptocytosis. Besides, sodium dodecyl sulphate-polyacrylamide gel electrophoresis of red blood cell membrane proteins revealed a significant reduction in protein 4.1 of 14% compared to control (Figure 2, C and D). Spectrin, ankyrin, protein 4.2, and band 3 proteins were quantitatively normal and had normal mobility patterns. As our patient had no evidence of familial history or previous signs of peripheral hemolysis, AE was the most likely in this context of newly diagnosed MDS.
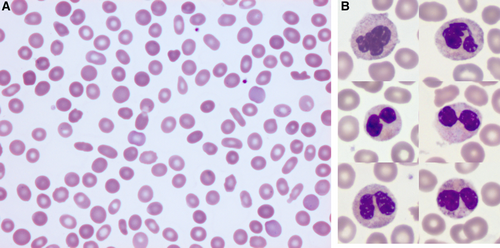
Blood cell morphology. Smear at medium (A) and high (B) magnification. (A), Frequent ovalocytes and rare elliptocytes are observed on the blood smear of this patient with acquired hemolytic anemia. May-Grünwald-Giemsa staining (×40). (B), Selected pictures of neutrophils showing normal aspect (top panel), hypolobulated aspect and/or hypogranulated aspect (medium and bottom panel). May-Grünwald-Giemsa staining (×100).
The patient was started on erythropoietin therapy and supportive transfusions. Two months later, 9% blasts were observed on peripheral blood smear, and a new bone marrow evaluation confirmed disease progression to MDS with excess blasts 1 with 6% blasts. Despite onset of treatment with 5-azacytidine, the disease rapidly progressed to acute myeloid leukemia and the patient died from cerebral hemorrhage 15 months after MDS diagnosis.
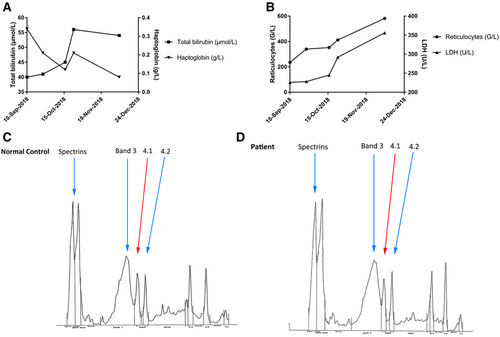
Exploration of hemolysis in the patient. (A), Evolution of reticulocytes (G/L, circles) and LDH (U/L, triangles) over time. (B), Evolution of total bilirubin (μmol/L, squares) and haptoglobin (g/L, triangles) over time. (C), Densitometry analysis of erythrocyte membrane protein electrophoresis in a normal control. (D), Densitometry analysis of erythrocyte membrane protein electrophoresis in our patient. For interpretation, the patient's protein 4.1 expression on gel electrophoresis was compared to the mean expression ±2 SD of 4 normal controls and was decreased by 14% compared to the control. In addition, we can observe that patient's protein 4.1 peak is decreased compared to protein 4.2, while both peaks are of similar intensity in the control (C). LDH = lactate dehydrogenase; SD = standard deviation.
Since its first description in 1984,5 around 20 cases of AE have been described.6 AE is most commonly observed in MDS patients, frequently associated with del(20q) but can also be observed in myeloproliferative neoplasms.7 Three quarters of reported MDS patients harbored at least 20q deletion, with constant dyserythropoiesis and dysmegakaryopoiesis, suggesting alteration of a common megakaryocyte/erythrocyte progenitor.8 We report here the first case of an AE in a MDS patient with isolated trisomy 8. Trisomy 8 is encountered in 3.8% to 5% of MDS9 and is generally associated with features of intermediate risk and prognosis.3 Strikingly, despite no previous report in a context of AE, this abnormality was described in 2 patients presenting with acquired spherocytosis that preceded the onset of MDS.10
Hereditary elliptocytosis (HE) is an autosomal disorder, more common in malaria-endemic regions in West Africa (1%-2%),11 caused by deficiency of either alpha spectrin, beta spectrin, protein 4.1, or glycophorin C. The majority of defects occur in spectrin, the principal structural protein of the erythrocyte membrane skeleton. Spectrin integrity is critical for erythrocyte membrane stability, shape, and function. Structural and functional defects of protein 4.1 appear to disrupt spectrin-actin interactions in the membrane skeleton.
The molecular mechanism of AE is unknown, but protein 4.1 deficiency by alteration of EPB41 gene expression (located on chromosome 1p) has been suggested, in line with diminished production of protein 4.1 observed in several cases.7 No abnormality of alpha or beta spectrin was ever reported in AE despite their frequency in HE. Some patients demonstrated normal expression of erythrocyte membrane proteins, which is more suggestive of a defect in the differentiation of an abnormal erythroid progenitor rather than a mechanism similar to that of HE. In our patient, we observed a significant decrease of protein 4.1 (-14%) in line with previous reports (-11% to -45%) in patients with AE.7
Haploinsufficiency of L3MBTL12 due to del(20q) was proposed as a possible mechanism explaining AE.6 The frequency, although likely underestimated, of AE in MDS patients with del(20q) is very low (less than 10% in our experience, congruent with a frequency of 5% of elliptocytosis reported by Mullier et al13), therefore we can hypothesize that another abnormality present only in a sub-population of MDS patients with del(20q) explains protein 4.1 deficiency and the observed AE.
The prognostic impact of AE in MDS remains unclear. Among the previously described cases of MDS-associated AE in the literature, there is only very few data on prognosis. Among the 7 cases with follow-up, including ours, 4 cases had a rapidly fatal evolution of their MDS.1, 6 Concerning our patient who had an intermediate prognosis according to Revised International Prognostic Scoring System, the presence of 5 different mutations, including ASXL1, TET2, and EZH2, could by itself be responsible for the rapid progression of the disease.4 Besides, although myeloproliferative features (apart from splenomegaly which could be in link with AE) were not present at MDS with multilineage dysplasia diagnosis, they appeared at the time of progression to MDS with excess blasts 1 (myelemia, hyperleukocytosis) and such patients have lower responses to hypomethylating agents and overall survival.14
In conclusion, AE should be evoked in MDS patients when hemolytic regenerative anemia is present and conversely, AE diagnosis should trigger additional explorations in patients and consideration of MDS diagnosis.
Disclosures
The authors declare no competing interest.