Challenging the Erythropoiesis Paradigm in β-Thalassemia
The authors have indicated they have no potential conflicts of interest to disclose.
β-thalassemia is a genetic disorder due to defective β-globin synthesis that results in severe anemia. A hallmark of the disease is the massive expansion of the erythroid compartment associated with enhanced but ineffective erythropoiesis. Whether this result in remodeling and homeostatic changes of the bone marrow (BM) niche and what are the consequences on disease pathophysiology is unclear and remains understudied. For many years, the BM microenvironment was considered an inert scaffold, providing structural support for hematopoietic stem and pro genitor cell (HSPC) contained within. In the past decade, however, the BM microenvironment has been described as a vibrant and complex living tissue with crucial homeostatic roles in hematopoiesis.1, 2
Along with cell-intrinsic factors, hematopoietic stem cell (HSC) self-renewal is maintained by extrinsic factors coming from the circulation and produced by the local microenvironment. Importantly, the BM microenvironment contributes to the maintenance of HSC homeostasis through a dynamic crosstalk with BM stromal cells (e.g. endothelial and mesenchymal stem cells, MSC). Although HSC transplant (HSCT) is an effective and currently the only curative approach for the correction of the erythropoietic defect, the risk of graft rejection in β-thalassemia is higher than in other indications for HSCT.3
A recent study by Aprile et al has brought the thalassemic BM niche into focus as potential explanation for limited HSCT success in this disease.1 By using the Hbb th3/+ (th3) mouse model that recapitulates the β-thalassemia phenotype, the authors demonstrated that th3 mice have both a reduced number and more active HSCs. th3 HSCs show a loss of quiescence and a higher cycling rate (Fig. 1). The elevated DNA damage and reduced clonogenic potential highlight the increased replication stress and impaired self-renewal ability of HSCs in β-thalassemia.
Through HSCT experiments, the authors demonstrated how an improved BM microenvironment can positively affect HSC behavior and ameliorate their functions.1 th3 HSCs transplanted in a recipient th3 BM show a competitive disadvantage compared to their co-transplanted WT counterparts. On the contrary, transplantation of th3 HSCs into a recipient wild-type BM restored HSC repopulating ability. Study of secondary transplants showed that th3 HSC transplant into wild-type recipients retained a long-term reconstitution ability, while their transplant into th3 recipients lead to progressive HSC exhaustion.
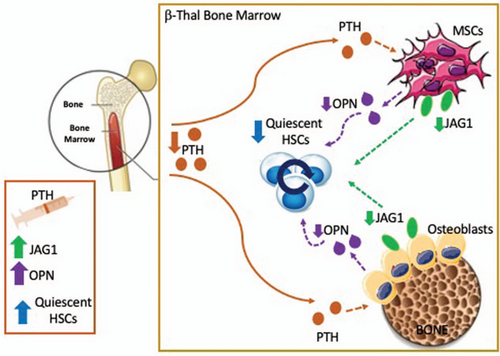
Role of impaired BM niche-HSC crosstalk in β-thalassemia. In β-thalassemia reduced circulating levels of the parathyroid hormone (PTH) negatively affects the BM microenvironment via altering number and functions of BM stromal cells, especially mesenchymal stem cells (MSC) and osteolineage cells, resulting in reduced bone density and defective HSC support. The reduced expression of the Notch-ligand JAG1 and OPN in thalassemic BM stromal cells lead to a defective activation of the Notch pathway and increased cycling activity of HSCs, thus affecting their self-renewal capacity and causing HSC pool exhaustion. PTH-based therapy is of benefit in β-thalassemia and results in the restoration of bone density as well as MSC number and functions, enhancement of JAG1 and OPN expression by the BM niche and improvement of HSC function.
To explain the disparity between transplant success, the authors next turned to the BM stromal niche. While there was no evident alteration in osteoblasts or osteoclasts, a decrease in bone network and mineral density, together with reduced type-I collagen levels were observed. Reduced serum alkaline phosphatase, osteopontin (OPN), and Notch-ligand Jagged 1 (JAG1) in th3 BM suggested altered osteoblast and MSC functions in β-thalassemia. Of note, OPN and JAG1 expression in osteoblasts and MSCs is dependent on the parathyroid hormone (PTH) and their reduction leads to a loss of HSC quiescence.4, 5 The PTH axis is a critical hormonal system that controls bone remodeling and also provides signals for HSC maintenance via its specific receptor (PTHR) on BM stromal cells. In β-thalassemia reduced circulating PTH levels likely contributes to a defective and poorly supportive BM microenvironment. The administration of PTH in th3 mice restored bone density as well as OPN and JAG1 levels and improves the pool of quiescent HSCs1 (Fig. 1).
In line with findings in the mouse model, patients with transfusion-dependent β-thalassemia (TDT) show reduced HSPC quiescence. Moreover, HSPC gene expression profile revealed upregulation of genes involved in cellular responses to oxidative stress and DNA damage as well altered BM collagen and JAG1 levels in TDT patients.1
Overall, these findings support the concept that, while HSCT is an effective strategy to cure β-thalassemia, its successful accomplishment also depends upon the improvement of the BM microenvironment.1, 2 BM niche alterations results in impaired niche-HSC crosstalk, leading to HSC exit from quiescence and premature HSC exhaustion in β-thalassemia. This suggests that HSCs develop impaired functions and loose stemness upon prolonged persistence in an altered BM microenvironment. Recent evidence indicates that stress signals, including oxidative stress, inflammation, iron, and hypoxia derived from ineffective erythropoiesis and chronic transfusions, may alter the BM niche, leading to MSC pool pauperization and defective microenvironmental support for HSC.2 Importantly, these observations indicate that the pathophysiology of β-thalassemia is not exclusively a dyserythropoiesis disorder, as the long-standing paradigm states, but a more complex disorder of hematopoiesis resulting from an impaired BM microenvironment and altered HSC functions.1, 2 These findings lay the groundwork for combined therapies which correct the erythropoietic defect and improve the BM microenvironment as well as HSC functions in pathologic conditions hallmarked by dyserythropoiesis. Targeting the BM microenvironment and correcting the HSC-stromal niche crosstalk will also provide a valuable strategy to improve transplantation and gene therapy approaches in these diseases.