High EVI1 Expression due to NRIP1/EVI1 Fusion in Therapy-related Acute Myeloid Leukemia: Description of the First Pediatric Case
Mariella D'Angiò and Grazia Fazio contributed equally to the manuscript.
The authors have no conflicts of interest to disclose.
Disruption of chromosome 3 at band 3q26 has been well-documented in acute myeloid leukemia (AML), chronic myeloid leukemia (CML) and myelodysplastic syndromes (MDS).1 Clinically, 3q26.2 rearrangements correlate with elevated platelet counts, marked hyperplasia with dysplasia of megakaryocytes, aggressive clinical course and unfavorable prognosis with conventional therapy.2, 3 Chromosome 3q26 abnormalities have been reported to activate the aberrant expression of the human Ecotropic Virus Integration site-1 gene (EVI1 known as MECOM-MDS1 and EVI1 complex locus), a transcriptional factor with an essential role in proliferation and maintenance of hematopoietic stem cells.4, 5 Although the exact role of EVI1 in leukemogenesis is not completely known, a recent study revealed that it may be involved in leukemic cell proliferation and apoptosis via the regulation of miR-9 promoter methylation, thus suggesting the possible role of hypomethylating agents in EVI1 over-expressed leukaemia.6-8 EVI1 up regulation occurs in approximately 8% to 10% of human adult AML and, strikingly, up to 27% of pediatric KMT2A (MLL) rearranged leukemia cases.9, 10 The most frequent abnormalities resulting in inappropriate activation of EVI1 are the inversion inv(3)(q21q26) and the translocation t(3;3)(q21;q26.2). However, EVI1 can be rearranged with a variety of other partner genes [1q41 (DUSP10), 7q21 (CDK6), 7q34 (TCRB), 12p34 (ETV6), 21q22 (RUNX1)].3, 11, 12 Its increased expression can also be detected in cytogenetically normal AML, in aberrant cytogenetic subgroups, such as monosomy 7 and 11q23/MLL translocations, as well as in patients with cryptic 3q26 rearrangements.3, 12 In particular, the cryptic t(3;21)(q26;q11) rearrangement, resulting in NRIP1/EVI1 fusion, has been identified using FISH analyses in 9 adults, 4 AML and 5 MDS so far.12 All patients showed a high EVI1 expression and an adverse prognosis with a median overall survival (OS) of 9.4 months.
Here we report the first pediatric case of therapy-related AML (t-AML) carrying a cryptic t(3;21)(q26;q11) rearrangement and EVI1 over-expression.
A previously healthy 4-year old child had been referred to our institution for cervical lymphadenopathy, peripheral cytopenia and spleen enlargement; peripheral blood (PB) smear and bone marrow (BM) aspiration revealed infiltration of lymphoid blast cells, cytogenetic analysis showed normal karyotype. The child was diagnosed with B-lineage acute lymphoblastic leukemia (ALL), treated according to AIEOP-BFM ALL2009 protocol and allocated to the high-risk arm, due to an insufficient molecular response [https://clinicaltrials.gov/ct2/show/study/NCT01117441].
Eight years after disease onset and 6 years after elective discontinuation of treatment, the patient experienced fatigue and her cell blood count (CBC) showed anemia, thrombocytopenia and circulating blasts (hemoglobin 8.8 g/dL, platelets 68 x 109/L, white blood cell count 8.8 x 109/L); morphological analyses detected 79% of undifferentiated blasts in PB and 60% in BM of undetermined FAB (French American British) classification (Fig. 1A). Cells were positive for CD45, CD34, CD117, HLA-DR, CD13, CD33, CD123, and MPO (Fig. 1B), consistent with a diagnosis of AML. Cytogenetic analysis, carried out on BM blasts by standard techniques and evaluated by Q banding, showed a complex karyotype with numerical and structural abnormalities (44,XX,-2,-3,del(5)(q22q35),-6,-7,del21(21)(q11),+2mar[19]/46,XX[1]) (Fig. 1C), thus including monosomy 7, known to be associated with poor prognosis.13 Target capture RNA NGS sequencing performed by TruSight Pancancer panel (Illumina, San Diego, CA, USA)14 revealed a fusion involving the NRIP1 (exon 2) and EVI1 (exon 2) genes, subsequently confirmed by RT-PCR and Sanger sequencing (Fig. 2A, B). EVI1 expression was evaluated by Real-time quantitative PCR (RQ-PCR) (Light Cycler 480 System Roche Diagnostics) and calculated by the comparative cycle time (DDCt) method with GUS as the housekeeping gene. Over-expression of EVI1 was found in the patient's BM compared to four healthy donors and three pediatric AML (2 de novo and 1 t-AML) negative for 3q26 rearrangements, whose BM were analyzed as controls (Fig. 2C). In order to exclude a clonal relationship between the ALL and AML diseases, the IG/TR rearrangements detected at ALL onset were evaluated on AML blasts; similarly, the NRIP/EVI1 fusion transcript, detected on AML blasts, was tested on ALL cells (Fig. 2A); both analysis were negative, revealing that the two leukemias have no clonal relation. Furthermore, to exclude an underlying Li Fraumeni syndrome associated to leukemia recurrence, TP53 sequencing was performed by NGS, and neither mutations nor copy number variation (CNV) were detected.
Conventional AML treatment was given, consisting of three sequential high-dose chemotherapy blocks (fludarabine, cytarabine and liposomal doxorubicin; mitoxantrone, etoposide and cytarabine; cladribine and cytarabine) usually used for AML; a progressive reduction of BM blast percentage was obtained but no CR was ever reached. A haploidentical hematopoietic stem cell transplantation (HSCT), after a treosulfan-based conditioning (treosulfan, fludarabine and thiotepa), was performed during post-chemotherapy pancytopenia 5 months after the diagnosis of AML. Ninety days after transplantation 2% atypical undifferentiated blasts and mixed chimerism (donor ≤95%) were detected in her BM. Neither a donor lymphocyte infusion (DLI) nor a dose of gentuzumab ozogamicin plus standard dose cytarabine were able to control the disease. Based on the potential efficacy of hypomethylating agents in EVI1 over-expressed leukemia,6-8 one cycle of azacitidine was given without any clinical or hematological benefits. The patient died due to disease progression 7 months after HSCT.
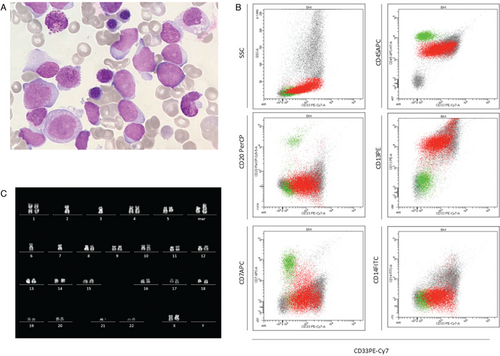
Morphology, immunophenotype and cytogenetic analyses of t-AML diagnosis. A. Image of bone marrow smear showing undifferentiated blast cells. B. Cytometry analysis is consistent with AML diagnosis. C. Karyotype analysis: G banding consistent with complex karyotype with numerical and structural abnormalities.
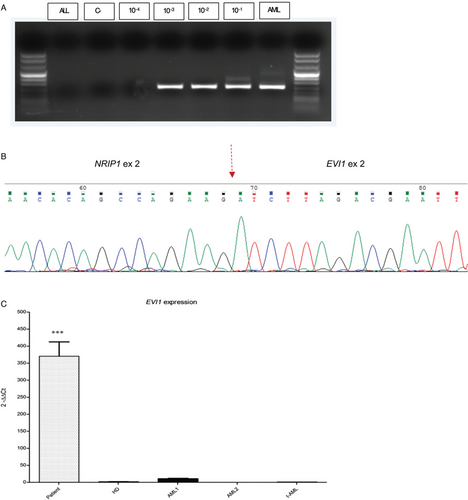
RT-PCR (A) and Sanger sequencing (B) of NRIP1/EVI1 fusion gene. (C) EVI1 expression in the patient with NRIP1/EVI1 fusion, pediatric de novo AML (n = 2), pediatric t-AML (n = 1) without any 3q26 rearrangement, healthy donor (n = 4). The patient showed statistically significant EVI1 over-expression compared to all other categories (RQ-PCR, ANOVA analysis, p < 0.0001).
The case here described is, to the best of our knowledge, the first reported pediatric patient affected by NRIP1/EVI1 fusion t-AML and EVI1 over-expression. Furthermore, it may be considered exceptional, due to the lowest reported frequency of pediatric tAML and of the NRIP1/EVI1 fusion transcript.
In fact, up to now, the diagnosis of t-AML has been reported in only 0.5% of children and young adults previously treated for cancer15 and NRIP1/EVI1 fusion has been described exclusively in 0.9% of adult AML-MDS patients.12
Consistent with our case, monosomy 7 has been frequently reported as associated to EVI1 rearrangements,12 and most adult cases have similarly shown a very aggressive clinical course as well as an inadequate response to conventional therapy.
Recent evidences showed that EVI1 may regulate the abilities of methyltransferase and acetyltransferase to modify gene expression via epigenetic mechanism; in particular, the down regulation of miR-9 may be involved in leukaemogenesis.8 These findings, worthy of further investigation, indicate the possibility of using hypomethylating agents in AML/MDS patients with NRIP1/EVI1 fusion transcript and/or EVI1 over-expression refractory to chemotherapy.
In patients with de novo or t-AML partially refractory to chemotherapy, it could be worth to search EVI1 rearrangements, even if cytogenetically cryptic, and/or to investigate EVI1 expression in order to consider including hypomethylating agents early in the treatment course.
Furthermore, with the aim to improve the poor prognosis reported in both EVI1 rearranged and/or over-expressed de novo and t-AML, joint cooperative efforts would be needed to investigate the role of azacitidine as additional or substitutive therapy to conventional schedules.