PB2224: ABBV-744 ALONE OR IN COMBINATION WITH RUXOLITINIB OR NAVITOCLAX IN PATIENTS WITH MYELOFIBROSIS: A PHASE 1B STUDY
Abstract Topic: 16. Myeloproliferative neoplasms - Clinical
Background: Myelofibrosis (MF) is a myeloproliferative neoplasm characterized by uncontrolled inflammation resulting in bone marrow fibrosis, splenomegaly, and constitutional symptoms. The Janus kinase inhibitors (JAKis) ruxolitinib and fedratinib are approved for the treatment of MF based on reduction of symptomatology and splenomegaly, however, not all patients respond. Furthermore, they do not appear to modify underlying disease biology, and survival benefit is minimal. Inhibitors of the bromodomain and extraterminal (BET) family of proteins (BETi) have shown the potential to modify disease biology in MF by inducing improvements in marrow morphology and reducing fibrosis. It is hypothesized that selective inhibition of specific bromodomains (BDs) of the BET family of proteins (BDI or BDII) may decrease off-target toxicities relative to pan-BETi. ABBV-744 is a small molecule BETi highly selective for BDII.
Aims: This study will investigate the safety, pharmacokinetics, and preliminary efficacy of ABBV-744 alone or in combination with B-cell lymphoma (BCL)-xL and BCL-2 inhibitor navitoclax, or ruxolitinib in patients with MF.
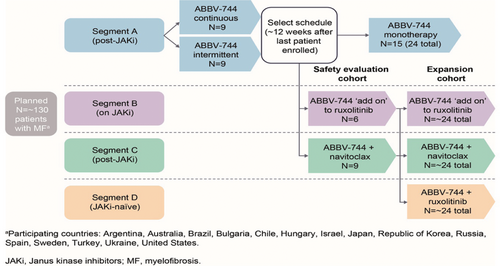
Methods: This Phase 1b multicenter, open-label study (NCT04454658) has a planned enrollment of approximately 130 patients across 60 sites worldwide. Eligible patients will be aged ≥18 years, with intermediate or high-risk MF, evidence of MF-related symptoms, Eastern Cooperative Oncology Group Performance Score ≤1, absolute neutrophil count ≥1500/mm3, platelets ≥100,000/mm3, and a creatinine clearance value of ≥50 mL/min. Exclusion criteria include concomitant CYP3A inhibitor use and prior exposure to BETi. The study will comprise 4 Segments (Figure 1). Segment A will assess ABBV-744 monotherapy at several dose levels with continuous or intermittent dosing, in patients with prior exposure to ≥1 JAKi. Using the optimal dosing regimen identified in Segment A, Segment B will assess ABBV-744 in combination with ruxolitinib (10 mg by mouth twice daily [BID]) in patients currently receiving ruxolitinib ≥10 mg BID, and with evidence of resistant or refractory disease. Segment C will investigate ABBV-744 combined with navitoclax (100–200 mg starting dose) in patients with prior exposure to ≥1 JAKi and resistant or refractory disease. Segment D will evaluate ABBV-744 combined with ruxolitinib in JAKi-naïve patients once the safety and efficacy in the JAKi-refractory population has been established (Segment B).
The primary endpoint is the proportion of patients with adverse events (graded per NCI CTCAE v5.0), including dose-limiting toxicities. Secondary endpoints include the proportion of patients achieving a reduction in spleen volume of ≥35% at Weeks 12 and 24, pharmacokinetic parameters including maximum observed plasma concentration (Cmax) and area under the plasma concentration-time curve, and the proportion of patients with a ≥50% reduction in total symptom score. Values for pharmacokinetic parameters will be determined using noncompartmental methods. Safety data will be summarized descriptively. Patients will continue treatment until progression or unacceptable toxicity, after which a 30-day safety follow-up visit will be conducted. Following disease progression, posttreatment follow-up visits will be conducted every 12 weeks. As of February 2023, 17 patients have been enrolled.
Figure 1. Study Schema
aParticipating countries: Argentina, Australia, Brazil, Bulgaria, Chile, Hungary, Israel, Italy, Japan, Republic of Korea,
Spain, Sweden, Turkey, United States.
JAKi, Janus kinase inhibitors; MF, myelofibrosis.
Results: Summary/Conclusion:
Keywords: Clinical trial, Myelofibrosis