P1303: DARATUMUMAB-BASED MAINTENANCE IN PATIENTS WITH RELAPSED MULTIPLE MYELOMA AFTER SALVAGE AUTOLOGOUS HEMATOPOIETIC STEM CELL TRANSPLANTATION
Abstract Topic: 22. Stem cell transplantation - Clinical
Background: Maintenance therapy is considered standard of care after upfront autologous hematopoietic stem cell transplantation (auto-HCT) for patients with newly diagnosed multiple myeloma (MM). However, there is paucity of data on the use of maintenance after a salvage auto-HCT, whether first or second.
Aims: We designed a clinical trial for relapsed MM patients who underwent salvage auto-HCT after disease relapse to receive post-transplant maintenance with daratumumab (dara), with the addition of pomalidomide (pom) in a later amendment.
Methods: We hypothesized that maintenance therapy with dara +/- pom will improve the progression-free survival (PFS) after a salvage auto-HCT. Patients with prior exposure to dara or pom were included, but patients refractory to either drug were excluded. The trial was planned to enroll a total of 56 patients. Patients received dara SQ weekly for weeks 1–8, every 2 weeks for weeks 9–24, and monthly from week 25 until progression. Pom was given at 2 mg PO from day 1–21 every 28 days. Patients received herpes zoster prophylaxis for the duration of the trial. The primary outcome of this phase II trial was PFS. Disease response was assessed according to the International Myeloma Working Group (IMWG) uniform response criteria. Minimal residual disease (MRD) was measured by multiparametric flow cytometry (10-5) in the bone marrow at day 90–180 after transplant.
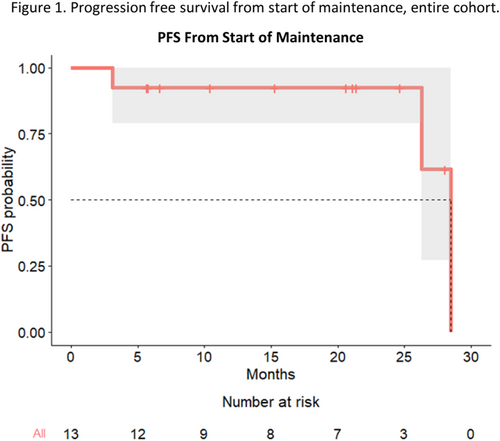
Results: The trial was terminated due to poor accrual after 13/56 patients were enrolled between May 2019 and August 2022. Median age at salvage auto-HCT was 64 (range: 43–76) years, and 7 patients (54%) were male. Median prior lines of treatment was 2 (range: 2–4). Six patients (46%) had a prior auto-HCT, and 7 (54%) had a hematopoietic stem cell comorbidity index (HCT-CI) of >3. Median time from diagnosis to salvage auto-HCT was 58.3 (range 7.6-–132) months. Five patients (38%) had high-risk cytogenetic abnormalities (1q+ in all 5). Eight (61%) patients received melphalan alone, 4 (31%) received busulfan/melphalan, and 1 (8%) received gemcitabine/busulfan/melphalan/panobinostat as their conditioning regimen (Table 1). Three patients (23%) started maintenance with dara alone, while the remaining 10 (77%) patients received dara + pom. At study entry, 6 patients (46%) were in complete response (CR), 4 (31%) in very good partial response (VGPR), 2 (15%) in partial response (PR), and 1 (8%) had stable disease (SD). At day 100 post maintenance, 9 (69%) were in CR, while 1 (8%) each had VGPR, PR, SD and progressive disease (PD), respectively. For the 12 patients that did not progress at day 100, best response to dara +/- pom maintenance was CR in 10 (83%), and VGPR and SD in 1 (8%) each. With a median follow up of 20.6 months (range: 5.7–43.9) from the start of maintenance, 3 patients had progressed but there are no deaths so far. Median PFS from the date of auto-HCT was 31.9 (31.1-NA) months, and from start of maintenance 28.5 (95% CI 26.3-NA) months. 2-year PFS was 92% (Figure 1). Most common adverse events were neutropenia (n=11, 84%; none grade>3), bacterial infection (n=6, 46%; none grade >3), and grade 1-2 diarrhea, grade 1-2 fatigue, grade 1 thrombocytopenia (n=5, 38% each).
Summary/Conclusion: Maintenance therapy with dara, with or without pom, is safe and feasible after a salvage auto-HCT for MM, with 83% achieving a CR. After a median follow up of 2 years, the median PFS was 28.5 months. The trial was terminated due to poor accrual.
Keywords: Autologous hematopoietic stem cell transplantation, Maintenance, Multiple myeloma, Stem cell transplant