P361: HIGH REMISSION AND SURVIVAL RATE IN ADULTS WITH MINIMAL RESIDUAL DISEASE-POSITIVE OR RELAPSED/REFRACTORY B-CELL ACUTE LYMPHOBLASTIC LEUKAEMIA TREATED WITH BLINATUMOMAB IN ROUTINE CLINICAL PRACTICE
Background: This prospective observational study included adult patients (pts) who received blinatumomab for treatment of minimal residual disease-positive (MRD+) or relapsed/refractory (R/R) Philadelphia chromosome-negative (Ph-) B-cell ALL (including a subset of pts with late first relapse [LFR]) in routine clinical practice in 11 European countries.
Aims: To describe 5-year interim analysis of effectiveness and safety data of blinatumomab in these pts.
Methods: Adult pts who initiated blinatumomab between 28 December 2015 and 22 March 2022 were included. Treating clinicians categorised the disease state of pts at blinatumomab initiation as MRD+ or R/R (including LFR pts, defined as first remission duration of ≥12 months [mos]). Pts were followed-up until data cut-off (24 May 2022), death, loss-to-follow-up or withdrawal, whichever came first. Survival outcomes are estimated using Kaplan Meier (KM) methodology. Disease-free survival (DFS) in MRD+ pts is defined as time from blinatumomab initiation to relapse (>5% bone marrow blasts or blasts in peripheral blood) or death. Relapse-free survival (RFS) in R/R pts is defined as time from complete haematologic remission with full/partial/incomplete recovery (CR/CRh/CRi) to relapse or death. Overall survival (OS) is defined as time from blinatumomab initiation to death of any cause.
Results: The analysis included 41 MRD+ and 158 R/R pts of whom 52 were LFR pts. Pts received a median of 2 cycles of blinatumomab.
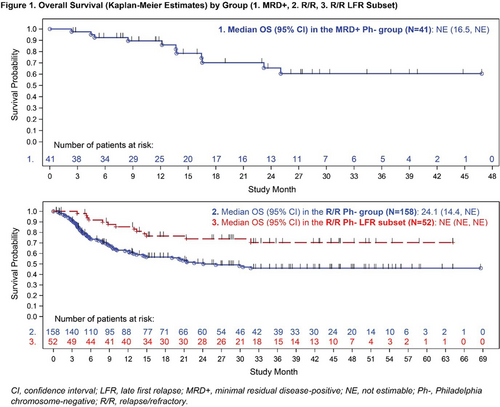
MRD+ pts’ median age was 41 years at blinatumomab initiation. At blinatumomab start, 70.7% (n=29) of patients were in first CR, 19.5% (n=8) and 4.9% (n=2) of pts received first or second salvage therapy, respectively. Three (7.3%) pts received prior haematopoietic stem-cell transplantation (HSCT). Within 2 blinatumomab cycles, among 31 pts evaluable for MRD, 67.7% (n=21) achieved a MRD response (MRD level <10-4). KM estimates for DFS and OS at 24 mos were 58.0% (95% confidence interval [CI]: 40-72) and 65.0% (95% CI: 44-80; Figure 1), respectively. Following blinatumomab treatment, 26 (63.4%) pts proceeded to allogeneic HSCT (alloHSCT).
R/R pts’ median age at blinatumomab initiation was 48 years (41 for LFR pts). Before blinatumomab start, 60.1% (n=95) of pts had not received prior salvage, first and second salvage was received by 24.1% (n=38), and 8.2% (n=13), respectively. Prior HSCT was described for 19.6% (n=31) of pts (including 25.0% [n=13] LFR pts). Within 2 blinatumomab cycles, 75.3% (n=119) of pts achieved CR/CRh/CRi, including 82.7% (n=43) of LFR pts. Out of pts in CR/CRh/CRi evaluable for MRD response, 61.3% (n=73/119; 65.1% [n=28/43] of LFR) achieved a MRD response. RFS and OS at 24 mos were 48.0% (95% CI: 38-57; LFR: 65% [49-78]) and 51.0% (95% CI: 42-59; LFR: 74.0% [59-84]; Figure 1) respectively. Almost two-thirds (62.2%, n=74) of pts in CR/CRh/CRi, including 65.1% (n=28) of LFR pts, proceeded to alloHSCT.
At data-cut-off, 61% (n=25) of MRD+ and 65.8% (n=104) of R/R pts including 71.2% (n=37) of LFR pts experienced treatment-related treatment-emergent adverse events (TEAEs). The most common (≥5%) serious TEAEs among the entire study population were cytokine release syndrome (8.4%) and neutropenia (5.4%).
Summary/Conclusion: In real-world clinical practice, survival outcomes confirm blinatumomab as an effective therapy in pts with MRD+ and R/R Ph- B-cell ALL including those with LFR. Within 2 blinatumomab cycles, high MRD response rates were observed. Over two-thirds of pts with R/R Ph- ALL achieved CR/CRh/CRi and 62.2% of those R/R pts proceeded to alloHSCT. Overall, the benefit-risk profile of blinatumomab remains favourable.
Keywords: Real world data, Relapsed acute lymphoblastic leukemia, MRD, Survival