S212: A RANDOMIZED DOUBLE-BLIND PHASE 3 STUDY OF JAKTINIB VERSUS HYDROXYUREA IN PATIENTS WITH INTERMEDIATE-2 OR HIGH RISK MYELOFIBROSIS
Background: Ruxolitinib and hydroxyurea are recommended by Chinese myelofibrosis guideline for splenomegaly. There is an unmet need for new treatments. Jaktinib, a novel JAK and AVCR1 inhibitor, showed promising activity on splenomegaly, anemia, and myelofibrosis symptoms in a phase 2 study (NCT03886415).
Aims: This randomized double-blind phase 3 study was aimed to assess the efficacy and safety of jaktinib compared to hydroxyurea in patients with intermediate-2 or high risk myelofibrosis.
Methods: Patients aged ≥18 with primary, post-polycythemia vera or post-essential thrombocythemia myelofibrosis, dynamic international prognostic scoring system (DIPSS) Int-2 or high risk, and no prior or ≤10 days’ treatment with a JAK inhibitor were enrolled and randomly assigned (2:1) to receive jaktinib 100 mg bid plus hydroxyurea placebo or hydroxyurea 0.5 g bid plus jaktinib placebo, stratified by DIPSS risk status (Int-2 or high risk). One interim analysis was pre-specified to be conducted when the 70 patients completed the 24-week treatment or met the criteria for the treatment termination before week 24. The primary endpoint was the proportion of patients with a spleen volume reduction of ≥35% from baseline (SVR35) at week 24, measured by MRI/CT images and assessed by Independent Review Committee. Secondary endpoints included the best spleen response rate (defined as achieving SVR35 at any time), the proportion of patients with a ≥50% reduction in Total Symptom Score (TSS50), improvement of anemia, safety, etc.
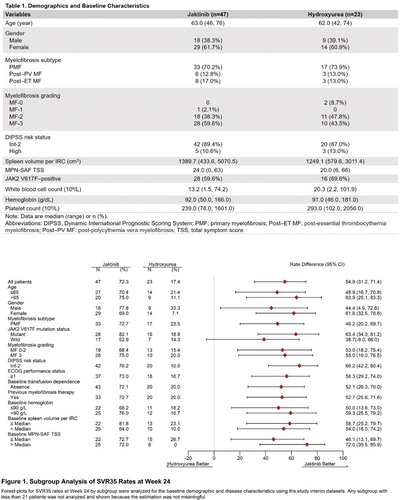
Results: This interim analysis (data cut-off: April 8, 2022) included 47 patients receiving jaktinib and 23 receiving hydroxyurea. 66.0% (jaktinib) and 69.6% (hydroxyurea) had a baseline hemoglobin <100 g/L. 59.6% (jaktinib) and 69.6% (hydroxyurea) were JAK2V617F positive. Patient baseline characteristics were well balanced between the two arms (Table 1).
The SVR35 rates at week 24 were 72.3% for jaktinib vs. 17.4% for hydroxyurea (p≤0.0001). Jaktinib showed a consistent spleen response benefit over hydroxyurea across all subgroups analyzed (Figure 1). The best spleen response rates were 80.9% of jaktinib-treat patients vs. 26.1% of hydroxyurea-treated patients (p≤0.0001). The median maximum percentage change from baseline in spleen volume were -46.59% vs. -18.50%.
The TSS50 rates at week 24 were 63.8% for jaktinib vs. 43.5% for hydroxyurea (p=0.1163). 5 of 7 jaktinib-treated and two of 5 hydroxyurea-treated patients who required red blood cell transfusion at baseline achieved a ≥50% decrease in red blood cell transfusion by week 24. One of four jaktinib-treated and 0 of three hydroxyurea-treated patients who were transfusion-dependent at baseline changed to transfusion-independent. In transfusion-independent patients with baseline hemoglobin ≤100 g/L, 39.3% for jaktinib and 15.4% for hydroxyurea had a ≥20 g/L hemoglobin increase.
The most common grade ≥3 hematological treatment-emergent adverse events (TEAEs) were anemia (25.5% [jaktinib] vs. 43.5% [hydroxyurea]), thrombocytopenia (17.0% vs. 39.1%), leukopenia (2.1% vs. 21.7%), neutropenia (2.1% vs. 21.7%) and decreased lymphocyte count (2.1% vs. 13.0%). Most common non-hematological TEAEs were upper respiratory tract infection (21.3% vs. 21.7%), elevated bilirubin (12.8% vs. 26.1%), fever (12.8% vs. 21.7%) and diarrhea (10.6% vs. 21.7%), predominantly of grade 1 or 2. TEAEs leading to treatment discontinuation occurred in 8.5% of jaktinib and 17.4% of hydroxyurea.
Summary/Conclusion: Jaktinib demonstrated significant clinical benefits over hydroxyurea in myelofibrosis patients for spleen response with improved symptom response and less cytopenias. Jaktinib may be a new treatment option for myelofibrosis patients, especially for those with anemia.
Keywords: Myeloproliferative disorder, Myelofibrosis, Janus Kinase inhibitor, Phase III