S159: HEALTH-RELATED QUALITY OF LIFE OF PATIENTS WITH CHRONIC MYELOID LEUKEMIA AFTER DISCONTINUATION OF TYROSINE KINASE INHIBITORS: RESULTS FROM THE EURO-SKI STUDY
Background: Stopping long-term therapy with tyrosine kinase inhibitors (TKIs) is now an option for an increasing number of patients with chronic phase chronic myeloid leukemia (CP-CML). However, there is paucity of evidence-based data on health-related quality of life (HRQoL) and symptom profile of these patients, once stopping therapy.
Aims: We herein report HRQoL results of the EURO-SKI international study (Saussele S, et al. Lancet Oncol. 19:747-757, 2018) by examining HRQoL trajectories over time by age groups.
Methods: The EURO-SKI was a prospective, open label, non-randomized trial enrolling adult patients with CP-CML across 11 countries in Europe. Patients had to be in treatment with any TKI for at least 3 years, and had to be in MR4 (BCR::ABL1 < 0.01% IS) for at least 1 year. HRQoL was a prespecified secondary endpoint of the study and was assessed with the EORTC QLQ-C30. A disease specific validated fatigue measure was also included: the FACIT-F. Evaluations were performed at baseline (at the time of treatment stop) and at month 1, 3, 6 and 12. As we hypothesized that benefits of TKI discontinuation would vary by age, the following four age group categories were considered: 18-39 (n=62 pts), 40-59 (n=272 pts), 60-69 (n=209 pts) and ≥70 (n=143 pts) years. A linear mixed effects model and growth curve analysis was used to analyze EORTC QLQ-C30 scores at baseline, month 1, month 3, month 6 and month 12 after TKI discontinuation. The longitudinal analysis for each age group modeled the different scores using fixed effects for the following variables: sex, Sokal-risk categories, MR4 duration in years, relapse, and times of visit. A random error was considered in the models to account for the within subjects’ design variability. We also analyzed the proportion of patients experiencing improvement, stability or deterioration at 6 and 12 months by using previously established scale specific criteria for the EORTC QLQ-C30.
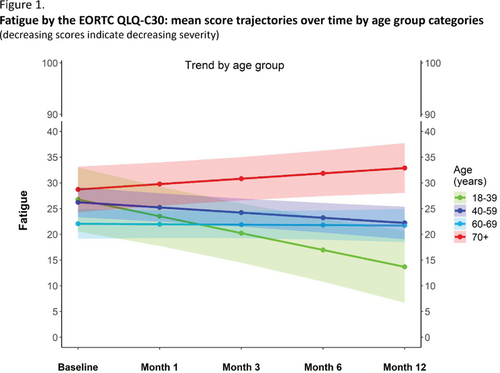
Results: Of the 728 evaluable patients at baseline, 686 (94%) had a HRQoL assessment completed. Median age at the time of stopping TKIs was 60 (range 18-89) years and these patients had a median duration of therapy of 7.6 years. Investigation of the mixed model adjusted HRQoL trajectories over time, revealed a different pattern of changes within each age group category. Younger patients (ie., aged between 18-39 years and 40-59 years) typically reported the greatest benefits across several functional and symptom outcomes. For example, there was a statistically significant decrease of burden of fatigue (FACIT-F) over time in patients aged between 18-39 years (p=.016) and in those aged between 40-59 years (p=.002). No statistically significant improvements were observed in the other two older age groups. This finding was corroborated by results of the fatigue scale of the EORTC QLQ-C30 (Figure 1). Inspection of the proportion of patients with clinically relevant changes (deteriorated, improved, or stable) from baseline to 6 and 12 months, also indicated differences by age groups. For example, in the group of patients with no relapse, the percentage of patients who reported deterioration in physical functioning between baseline and 6 months was: 16%, 44%, 47% and 65%, in the groups aged 18-39, 40-59, 60-69 and ≥70 years, respectively.
Summary/Conclusion: This large international study, suggests that HRQoL advantages of stopping long-term TKI therapy vary across different age groups, with younger patients benefiting the most. This information complements current knowledge in this area, and will help patients and physicians to make more informed decisions.
Keywords: Chronic myeloid leukemia, Quality of life, Patient reported outcomes