S121: REDUCED RATE OF VASO-OCCLUSIVE CRISES (VOCS) IN PATIENTS (PTS) WITH SICKLE CELL DISEASE (SCD) TREATED WITH CRIZANLIZUMAB FOR 12 MONTHS: RESULTS FROM A REAL-WORLD, MANAGED ACCESS PROGRAM (MAP)
Background: VOCs, the hallmark of SCD, can lead to serious complications and organ damage.1,2 The cell adhesion protein P-selectin plays a key role in the multicellular interactions that can lead to VOCs.3–5 Crizanlizumab is a first-in-class humanized monoclonal antibody that blocks P-selectin, and is approved in several regions to prevent/reduce VOCs for SCD pts aged ≥16 years. Since June 2018, SCD pts in some countries have been able to obtain early access to crizanlizumab before health authority approval via a MAP (NCT03720626).
Aims: To describe the rate of home- and healthcare-managed VOCs and use of opioids for VOC-related pain relief pre- and post-crizanlizumab initiation in SCD pts who participated in the MAP (in countries where publication of these data is allowed).
Methods: The MAP provided access to crizanlizumab for pts with serious or life-threatening disease (SCD) for which no comparable or satisfactory alternative to crizanlizumab was available as treatment in their country. Other eligibility criteria included: aged 16–70 years (18–70 years in Italy); history of VOCs as determined by the treating physician (including recurrent VOCs while taking preventative therapies eg hydroxyurea [HU]); and ineligibility for a crizanlizumab clinical trial. VOC and opioid use frequency are described for pts with available data 12 months pre-crizanlizumab initiation and after ≥12 months of treatment with crizanlizumab, overall and stratified by SCD genotype and history of HU use.
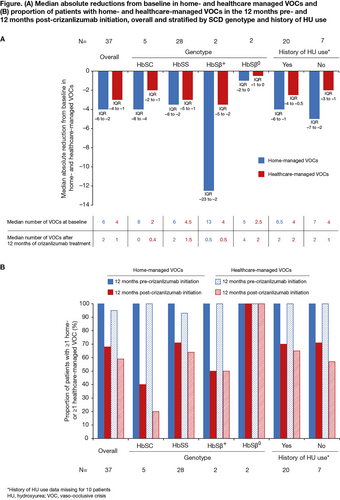
Results: As of October 2021, 37 pts had been treated with crizanlizumab for ≥12 months in the MAP; 34 (92%) in Brazil, two (5%) in Italy and one (3%) in Israel. Median (interquartile range [IQR]) age was 33 (25–40) years, 54% were female, 32% were African American, 3% Caucasian, 19% Hispanic and 46% of ‘other’ ethnicity, and 76% had the HbSS genotype. Data about HU use were only available for 27/37 pts in this analysis; 20 (74%) had a history of HU use. In the 12 months pre-crizanlizumab initiation, 76% (n=28/37) of pts were hospitalized for SCD-related complications (total: 89 events).
In the 12 months pre-crizanlizumab initiation (baseline), all 37 pts (100%) had ≥1 home-managed VOC and 95% had ≥1 healthcare-managed VOC. In the 12 months post-crizanlizumab initiation, 68% and 59% of pts had ≥1 home- and ≥1 healthcare-managed VOC, respectively. Median (IQR) absolute reductions from baseline in home- and healthcare-managed VOCs after ≥12 months of crizanlizumab treatment were –4.0 (–6.0 to –2.0) and –3.0 (–4.0 to –1.0), respectively. The lower rates of VOCs post- vs pre-crizanlizumab were also observed when stratifying the data by SCD genotype/prior HU use (Figure). Of note, a small number of pts are included in some groups when stratifying the data.
Opioids were taken for VOC management by 95% of pts (n=35/37) in the 12 months before crizanlizumab initiation and by 68% (n=25/37) in the 12 months after; the most common opioid taken was morphine (74% [n=26/35] and 56% [n=14/25], respectively).
Summary – Conclusion: Pts participating in the crizanlizumab MAP had a high burden of home- and healthcare-managed VOCs, as well as SCD-related complications at baseline, despite many pts reporting a history of HU use. Most pts reported using opioids for VOC management. Crizanlizumab substantially reduced the median annualized rates of home- and healthcare-managed VOCs and use of opioids from baseline in this real-world setting, consistent with results from SUSTAIN.6
References
1. Ballas & Lusardi. Am J Hematol 2005; 79:17–25
2. Herquelot et al, Value Health 2019; 22:S908; presented at ISPOR 2019
3. Zhang et al, Blood 2016; 127:801–9
4. Merle et al, JCI Insight 2018; 3:e96910
5. Kappelmayer & Nagy. Biomed Res Int 2017:6138145
6. Ataga et al, N Engl J Med 2017; 376:429–39