S108: A SYSTEMATIC POST-MARKETING (PM) REVIEW OF RARE INFUSION-RELATED REACTIONS (IRRS) PRESENTING AS PAIN EVENTS DURING OR AFTER CRIZANLIZUMAB INFUSION IN PATIENTS WITH SICKLE CELL DISEASE (SCD)
Background: Vaso-occlusive crises (VOCs) are the hallmark of SCD and can lead to complications and premature death. Crizanlizumab, an anti-P-selectin monoclonal antibody (mAb), is authorized to prevent/reduce VOCs in SCD patients aged ≥16 years.
IRRs are defined as signs/symptoms experienced by patients during/within 24 hours of infusion of a pharmacologic/biologic agent.1 IRRs are quite common with mAbs (frequency 1.6–99%)2 and can present as pain events, such as back pain, chest pain and myalgia. Pain events are known adverse drug reactions in the crizanlizumab label, however, pain events occurring during/within 24 hours of crizanlizumab infusion in SUSTAIN were not identified as potential IRRs.3 For SCD patients, IRR-related pain events may differ in location, severity and/or nature from their usual SCD/VOC pain.
Aims: We reviewed PM data on IRRs presenting as pain events in SCD patients treated with crizanlizumab.
Methods: A custom search of the Novartis safety database (comprising PM reports [spontaneous] and managed access/patient orientation program reports) was performed for reports received in November 2019–June 2021, using ~111 MedDRA terms associated with potential signs/symptoms of IRRs presenting as pain events. IRRs must have occurred during/within 24 hours of the most recent crizanlizumab infusion, and pain could differ from a patient's usual SCD/VOC. As reports were not gathered via a uniform data collection system, potential limitations include underreporting, incompletely documented cases, or bias towards reporting severe events.
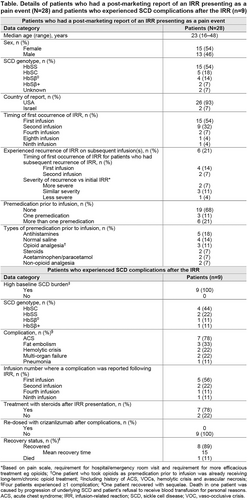
Results: IRRs presenting as pain events were experienced by 28 patients (Table), and most commonly presented as back pain, pain in extremity, arthralgia, musculoskeletal chest pain and headache. Reporting rate was 1.67 cases per 100 patient-years (95% CI 1.11–2.42). Most patients (n=24) initially experienced IRR at the first or second infusion. IRR recurred on subsequent infusion(s) in six patients. Twenty patients (71%) were hospitalized for treatment, including analgesics, antihistamines, IV fluids and/or steroids. Nine patients (32%) reported known complications of SCD following IRR (Table). Crizanlizumab was discontinued in 23 patients (82%) after their most recent IRR, including all who experienced secondary SCD complications.
All patients recovered (the majority within 3 days; one with sequelae), except one who died following SCD complications and refusal of blood transfusions for personal reasons. Resolution time was prolonged for patients who reported SCD complications following IRR. Causal analysis of complications was confounded by the underlying disease and use of steroids to treat IRR (systemic corticosteroid exposure in SCD patients has been associated with pain, complications [including severe VOCs and hemorrhagic stroke] and death). We do not know whether the 28 patients had an active VOC or other SCD complications before receiving crizanlizumab.
Summary – Conclusion: Although rare, based on review of PM data, healthcare professionals should be aware of the possibility of IRRs presenting as pain events during/after crizanlizumab infusion. Crizanlizumab labels have been/are being updated by Novartis to provide information on monitoring, management and prevention of IRRs, including a statement recommending caution when using corticosteroids. Given the limited data available regarding IRRs, Novartis is committed to further understanding these events.
References
1. Kang & Saif. J Support Oncol 2007; 5:451–7
2. Rombouts et al, Anticancer Res 2020; 40:1201–18
3. Ataga et al, N Engl J Med 2017; 376:429–39