S825 EVALUATION OF AMG 420, AN ANTI-BCMA BISPECIFIC T-CELL ENGAGER (BITE®) IMMUNOTHERAPY, IN R/R MULTIPLE MYELOMA (MM) PATIENTS: UPDATED RESULTS OF A FIRST-IN-HUMAN (FIH) PHASE 1 DOSE ESCALATION STUDY
Abstract
Background:
BCMA, a member of the TNFR family, is expressed on MM and plasma cells (PC).
Aims:
Objectives of this study included assessing safety and activity of AMG 420/BI 836909, which binds BCMA (B-Cell Maturation Antigen) on MM cells and CD3 on T cells, in relapsed and/or refractory (R/R) MM.
Methods:
In this FIH study (NCT02514239), 6-week cycles of AMG 420 were given for ≤5 cycles or until disease progression (PD), toxicity, or consent withdrawal; 5 more cycles could be given for benefit. Eligible patients had progression after ≥2 lines (incl PI and IMiD). Excluded were PC leukemia, extramedullary relapse, CNS involvement, or prior allo-SCT. MRD was defined as <1 tumor cell / 104 bone marrow cells per flow cytometry.
Results:
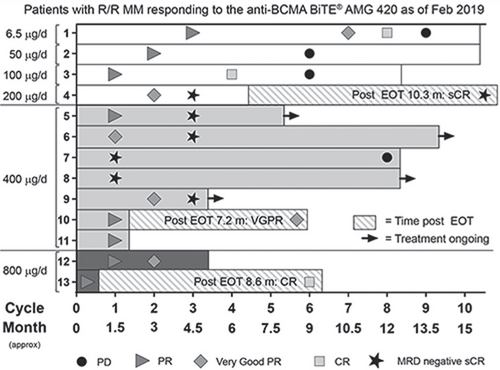
As of Dec 10, 2018, 42 patients received AMG 420 (0.2–800 μg/d). Patients D/C for PD (n = 24), adverse events (AE, n = 7, incl 3 DLTs), death (4), completed 10 cycles (2), and consent (1). Median age was 65 y, median MM duration 5.2 y, and median # prior therapies 4. Patients were treated for a mean (SD) of 2.5 (2.6) cycles.
There were 2 deaths from AEs (acute respiratory distress from flu / aspergillosis; fulminant hepatitis related to adenovirus infection); neither treatment related. Of those with serious AEs (SAEs, n = 21, 50%), 18 required hospitalization. SAEs occurring in >1 patient were infections (n = 12) and polyneuropathy (PN, n = 2). Treatment-related SAEs included 2 grade 3 PNs and 1 edema. Grade 2–3 CRS was seen in 3 patients. No anti-AMG 420 Ab were detected. In this study, 800 μg/d was determined to not be tolerable as 2/3 patients had DLTs, 1 case of grade 3 CRS and 1 case of grade 3 PN; both required hospitalization and subsequently resolved.
At 400 μg/d, there were 5 minimal residual disease (MRD)-negative sCRs, 1 VGPR, and 1 PR, for a response rate of 7/10 (70%); at Dec datacut, responses lasted for 5.6–10.4 months with 4 patients ongoing on treatment. As of Feb 2019, some responses lasted >1 year. Overall, there were 13/42 responders (6 sCRs, 3 CRs, 2 VGPRs, 2 PRs). Median time to any response was 1 month, with 9 of 13 patients responding in the first cycle.
Summary/Conclusion:
In this FIH study of AMG 420, a BiTE® vs BCMA, in R/R MM, there was a 70% response rate (7/10) with 5 out of 7 responders achieving a sCR at 400 μg/d, a recommended dose for further investigation.