Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12 440 patients of the ESC Heart Failure Long-Term Registry
Abstract
Aims
To evaluate how recommendations of European guidelines regarding pharmacological and non-pharmacological treatments for heart failure (HF) are adopted in clinical practice.
Methods and results
The ESC-HF Long-Term Registry is a prospective, observational study conducted in 211 Cardiology Centres of 21 European and Mediterranean countries, members of the European Society of Cardiology (ESC). From May 2011 to April 2013, a total of 12 440 patients were enrolled, 40.5% with acute HF and 59.5% with chronic HF. Intravenous treatments for acute HF were heterogeneously administered, irrespective of guideline recommendations. In chronic HF, with reduced EF, renin–angiotensin system (RAS) blockers, beta-blockers, and mineralocorticoid antagonists (MRAs) were used in 92.2, 92.7, and 67.0% of patients, respectively. When reasons for non-adherence were considered, the real rate of undertreatment accounted for 3.2, 2.3, and 5.4% of the cases, respectively. About 30% of patients received the target dosage of these drugs, but a documented reason for not achieving the target dosage was reported in almost two-thirds of them. The more relevant reasons for non-implantation of a device, when clinically indicated, were related to doctor uncertainties on the indication, patient refusal, or logistical/cost issues.
Conclusion
This pan-European registry shows that, while in patients with acute HF, a large heterogeneity of treatments exists, drug treatment of chronic HF can be considered largely adherent to recommendations of current guidelines, when the reasons for non-adherence are taken into account. Observations regarding the real possibility to adhere fully to current guidelines in daily clinical practice should be seriously considered when clinical practice guidelines have to be written.
Introduction
The therapeutic approaches for hospitalized patients with heart failure (HF) have remained practically unchanged during the last decades. Mainly for this reason, both in-hospital and 1-year outcomes of patients admitted for acute HF are still unacceptably high.1,2 In contrast, survival of patients with chronic HF seems to improve slowly over time,1–4 due to the fact that several trials have been conducted in these patients, allowing the inclusion of effective treatments in the recommendations of current guidelines.5–8 However, several studies showed that treatment guidelines are adopted slowly or are applied inconsistently, often failing to lead to further improvements in patient care quality and outcomes.9–13
The ESC-HF Pilot Survey provided a clear picture on the rate of use of guideline-recommended, evidence-based treatments.14 The rate of use of renin–angiotensin–aldosterone system blockers (ACE inhibitors, ARB, and aldosterone blockers) and beta-adrenergic blockers was satisfactory. However, the number of patients treated with appropriate doses was, at best, suboptimal. With respect to the rate of implantation of devices [CRT devicesand/or implantable cardioverter defibrillators (ICDs)], only a third of patients with the ICD characteristics were actually implanted and one-fifth were treated with CRT.14 This observation clearly confirmed the relevant gap between recommendations and actual clinical practice.15 Along the same lines, the EUROMED Registry showed that the implantation rates of ICD and CRT have increased significantly from 2004 to 2008, but underutilization was still present, with major differences across countries.16
For all these reasons, one of the major aims of the ESC Long-Term Registry was to evaluate how recommendations of most recent European guidelines regarding pharmacological and non-pharmacological treatments are adopted in clinical practice. More specifically, information on the reasons why evidence-based treatments are not utilized or underdosed with respect to the dosages recommended by guidelines have been collected and reported here.
Methods
Study design and clinical setting
The ESC-HF Long-Term Registry is a prospective, multicentre, observational study of patients presenting to 211 Cardiology Centres of 21 European and Mediterranean countries which are members of the European Society of Cardiology (ESC). Table S1 in the Supplementary material reports the name of the countries, their geographical area, and the number of recruited patients split into hospitalized and ambulatory patients with chronic HF.
The national cardiology societies of each country agreed to participate in the programme and were asked to select hospitals of different levels of complexity, from which patients would be recruited. The aim was to involve a broad spectrum of cardiology and/or HF units following outpatients with HF and admitting patients with acute, pre-existing, or new-onset HF to build up a network of centres that would be representative of the reality in Europe.
The number of participating centres for each country was decided according to the number of inhabitants in that country. As far as possible, the centres would also represent a balanced proportion across a different range of facilities for cardiology.
To facilitate consecutive enrolment, patients were enrolled in the registry on a ‘one day per week’ basis for 12 consecutive months in each participating country.
The EURObservational Research Programme (EORP) department at European Heart House was appointed to coordinate the project operationally, provide support to the committees, National Coordinators, and participating centres, and to guard the methodological concepts of the survey. The database was set up at European Heart House according to the requirements defined by the appointed Executive Committee, with the support of the EORP department.
Patient population
All outpatients with HF seen at the clinics, as well as those admitted for acute, pre-existing, or new-onset HF, were included during the enrolment period (1 day per week for 12 consecutive months). Therefore, during the course of the screening day, the following patients were entered in the survey:
- all outpatients with chronic HF diagnosed according to the clinical judgement of the responsible cardiologist at the participating centres;
- patients admitted to hospital for acute HF, for whom i.v. therapy (inotropes, vasodilators, or diuretics) was needed.
There were no specific exclusion criteria, with the exception that all patients must be aged over 18 years.
The survey was approved by each local Institutional Review Board according to the rules of each participating country. No data were collected before detailed information was provided to the patient, and a signed, informed consent was obtained.
Statistical analysis
Continuous variables are reported as median and interquartile range (IQR). Categorical variables are reported as percentages and compared using the χ2 test. Continuous variables are compared by the t-test or the Mann–Whitney U-test. A P-value of 0.05 was considered statistically significant. All tests were two-sided. Analyses were performed with SAS system software (SAS Institute, Inc., Cary, NC, USA).
Results
Figure S1 in the Supplementary material shows patient disposition of the ESC-HF Long-Term Registry. From May 2011 to April 2013, of the 12 785 patients screened for the study, 12 440 gave their informed consent and therefore are part of this analysis. Of these patients, 5039 (40.5%) were patients hospitalized for acute HF, while 7401 (59.5%) were ambulatory patients with chronic HF.
Patient characteristics
In-hospital patients were generally older than ambulatory patients with chronic HF and were more often female (Table 1). Co-morbidities were more frequent in patients admitted for acute HF, whereas the rate of implanted devices was significantly more common in patients with chronic HF. More than half of the patients with acute HF had an ischaemic aetiology, while in patients with chronic HF an ischaemic aetiology accounted for just 43% of the cases. Hospitalized patients with acute HF also showed a more severe profile in terms of laboratory measures: a haemoglobin level < 12 g/dL, higher creatinine and uric acid values, proteinuria, and higher BNP or NT-proBNP levels (when available) were detected much more frequently in hospitalized patients with acute HF than in those with chronic HF (Supplementary material, Table S2).
| HHF (n = 5039) | CHF (n = 7401) | P-value | |
|---|---|---|---|
| Age (years), median (IQR) | 71 (61–79) | 66 (57–75) | <0.0001 |
| ≥75 years, % | 39.5 | 26.0 | <0.0001 |
| Females, % | 37.3 | 28.8 | <0.0001 |
| BMI (kg/m2), median (IQR) | 28 (25–31) | 28 (25–31) | 0.0002 |
| SBP (mmHg), median (IQR) | 130 (110–150) | 120 (110–136) | <0.0001 |
| SBP ≤110 mmHg, % | 27 | 31 | <0.0001 |
| HR (b.p.m.), median (IQR) | 88 (73–104) | 70 (62–80) | <0.0001 |
| HR ≥70 b.p.m., % | 83.0 | 55.6 | <0.0001 |
| EF (%), median (IQR)a | 38 (30–51) | 35 (28-45) | <0.0001 |
| EF >45%, % | 32.8 | 23.1 | <0.0001 |
| NYHA III–IV, % | 85.9 | 25.3 | <0.0001 |
| Pulmonary or peripheral congestion, % | 85.0 | 74.7 | <0.0001 |
| Third heart sound, % | 32.5 | 5.8 | <0.0001 |
| Peripheral hypoperfusion/cold, % | 16.5 | 3.5 | <0.0001 |
| Mitral regurgitation, % | 44.4 | 26.2 | <0.0001 |
| Aortic stenosis, % | 9.4 | 3.9 | <0.0001 |
| Prior hospitalization, % | 29.8 | 40.9 | <0.0001 |
| HF diagnosis >12 months, % | 54.5 | 63.9 | <0.0001 |
| Ischaemic aetiology, % | 54.0 | 43.0 | <0.0001 |
| Atrial fibrillation, % | 44.0 | 37.6 | <0.0001 |
| Diabetes mellitus, % | 38.9 | 31.8 | <0.0001 |
| PAD, % | 14.2 | 12.3 | 0.0021 |
| Hypertension, % | 64.5 | 58.2 | <0.0001 |
| COPD, % | 20.2 | 13.8 | <0.0001 |
| Sleep apnoea, % | 3.0 | 5.2 | <0.0001 |
| Prior stroke/TIA, % | 13.0 | 9.4 | <0.0001 |
| Renal dysfunction, % | 26.4 | 18.2 | <0.0001 |
| Hepatic dysfunction, % | 8.4 | 3.4 | <0.0001 |
| Depression, % | 7.9 | 7.6 | 0.553 |
| PM, % | 6.2 | 5.8 | <0.0001 |
- a BMI, body mass index; CHF, chronic heart failure; HF, heart failure; HHF, hospitalized heart failure; HR, heart rate; IQR, interquartile range; PAD, peripheral artery disease; PM, pacemaker; SBP, systolic blood pressure; TIA, transient ischemic attack.
- a Available for 9722 patients.
With the exception of the ECG and echocardiogram, performed in > 80% of both hospitalized and ambulatory patients, the other investigations/procedures were performed quite infrequently, and generally at a higher rate in outpatients with HF (Supplementary material, Table S3). Table S4 in the Supplementary material shows the electrocardiographic and echocardiographic findings. Atrial fibrillation and LV hypertrophy (LVH) were reported more frequently in hospitalized patients, while a larger QRS was observed in outpatients (Supplementary material, Table S4A).
Patients with HF and preserved EF, defined as an EF >45%, accounted for 31.2% and 23.8% in hospitalized and ambulatory HF patients, respectively. LVH, moderate to severe mitral regurgitation, tricuspid regurgitation, and aortic stenosis were reported more frequently in hospitalized than in ambulatory patients (Supplementary material, Table S4B).
Pharmacological treatments of hospitalized patients with acute heart failure
The use of i.v. treatments for HF (diuretics, nitrates, and inotropic agents) split by the level of systolic blood pressure (SBP) at entry, as recommended by the ESC guidelines available at the time of patient recruitment,5 are reported in Table 2. The great majority of patients had an SBP at entry > 100 mmHg, while just a minority (2.4%) had an SBP < 90 mmHg. A relevant number of patients were treated with inotropes even with SBP values > 90 mmHg. The same observation was present for nitrates: > 23% of cases received an i.v. nitrate even with SBP values < 100 mmHg.
| According to 2012 ESC guidelines6 | ||||
|---|---|---|---|---|
| Total (n = 5039) | <85 mmHg (n = 90) | 85–110 mmHg (n = 1169) | >110 mmHg (n = 3484) | |
| I.v. inotropes, % | 11.9 | 73.3 (66 pts) | 22.3 (260 pts) | 6.8 (236 pts) |
| I.v. nitrates, % | 20.4 | 10.0 (9 pts) | 13.3 (155 pts) | 23.0 (798 pts) |
| I.v. diuretics, % | 81.5 | 77.8 (70 pts) | 82.9 (966 pts) | 81.1 (2814 pts) |
| According to 2008 ESC guidelines5 | ||||
| Total (n = 5039) | <90 mmHg (n = 117) | 90–100 mmHg (n = 539) | >100 mmHg (n = 4087) | |
| I.v. inotropes, % | 11. 9 | 70.1 (82 pts) | 29.9 (161 pts) | 7.8 (319 pts) |
| I.v. nitrates, % | 20.4 | 12.0 (14 pts) | 11.2 (60 pts) | 21.9 (888 pts) |
| I.v. diuretics, % | 81.5 | 78.6 (92 pts) | 83.1 (446 pts) | 81.4 (3312 pts) |
- a ppts, patients.
- a For 296 patients systolic blood pressure at entry was not reported.
Table 3 reports the use of oral recommended treatments prior to admission and at discharge of hospitalized patients with HF. These data show a clear, significant increase in the rate of prescription of all recommended treatments at discharge, with respect to the period preceding admission.
| Prior to hospitalization (n = 5039) | At discharge (n = 5039) | P-value | |
|---|---|---|---|
| ACE-I/ARBs, % | 64.3 | 77.0 | <0.0001 |
| Beta-blockers, % | 54.8 | 71.8 | <0.0001 |
| MRAs, % | 33.9 | 55.3 | <0.0001 |
| Diuretics, % | 65.3 | 83.6 | <0.0001 |
| Digitalis, % | 19.5 | 26.4 | <0.0001 |
| Statins, % | 42.6 | 58.4 | <0.0001 |
| Antiplatelets, % | 49.2 | 61.9 | <0.0001 |
| OAC, % | 30.8 | 42.3 | <0.0001 |
| Amiodarone, % | 8.9 | 13.7 | <0.0001 |
| Ivabradine, % | 1.2 | 3.2 | <0.0001 |
| Nitrates, % | 25.2 | 32.0 | <0.0001 |
| Calcium channel blockers, % | 15.8 | 15.9 | 0.59 |
- a ACE-I, ACE inhibitor; MRAs, mineralocorticoid receptor blockers; OAC, oral anticoagulant.
Pharmacological treatments of ambulatory patients with chronic heart failure
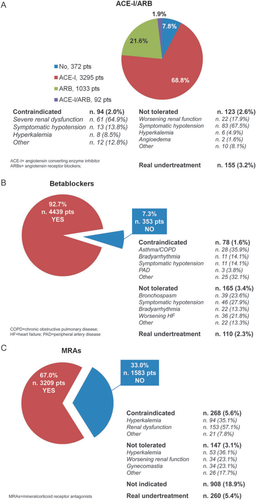
A blocker of the renin–angiotensin system, beta-adrenergic blockers, and mineralocorticoid antagonists (MRAs) were prescribed in 89.2, 88.9, and 59.3% of the cases, respectively (Table 4). Considering just the patients with reduced EF, for whom these drugs are recommended by guidelines, the rate of use of ACE inhibitors/ARBs, beta-blockers, and MRAs was 92.2, 92.7, and 67.0%, respectively. The reasons for non-prescription in patients with reduced EF are reported in Figure 1. Table S5 in the Supplementary material reports this information for the total population of patients and split by reduced and preserved EF. In the large majority of cases, the reported contraindications or a documented intolerance were the reasons for non-prescription of these drugs.
| Treatments | Total population (n = 7041), % | Reduced EF (≤45%) (n = 4792), % | Preserved EF (>45%) (n = 1499), % |
|---|---|---|---|
| ACE-I/ARBs | 89.2 | 92.2 | 79.7 |
| ACE-I | 67.0 | 70.7 | 55.4 |
| Ramipril | 43.9 | 45.8 | 37.9 |
| Enalapril | 27.5 | 27.2 | 27.7 |
| Perindopril | 14.5 | 12.6 | 21.9 |
| Beta-blockers | 88.9 | 92.7 | 78.8 |
| Carvedilol | 40.7 | 40.6 | 31.9 |
| Bisoprolol | 40.8 | 42.0 | 44.9 |
| Metoprolol | 11.0 | 10.7 | 12.2 |
| ARBs | 23.9 | 23.5 | 25.9 |
| Candesartan | 27.8 | 31.6 | 19.1 |
| Losartan | 27.0 | 27.3 | 24.5 |
| Valsartan | 30.0 | 28.6 | 36.9 |
| MRAs | 59.3 | 67.0 | 40.8 |
| Spironolactone | 68.3 | 66.7 | 74.6 |
| Eplerenone | 23.7 | 24.5 | 17.8 |
| Canrenone | 4.3 | 4.6 | 4.1 |
| Diuretics | 83.1 | 84.3 | 78.5 |
| Digitalis | 23.0 | 23.9 | 19.0 |
| Statins | 60.9 | 61.8 | 55.6 |
| Antiplatelets | 48.7 | 51.5 | 40.4 |
| Oral anticoagulant | 42.4 | 41.9 | 45.6 |
| Amiodarone | 13.8 | 14.7 | 10.2 |
| Ivabradine | 8.5 | 10.5 | 4.9 |
| Nitrates | 19.4 | 18.3 | 19.4 |
| Calcium channel blockers | 11.3 | 7.4 | 21.1 |
- a ACE-I, ACE inhibitor; MRAs, mineralocorticoid receptor blockers.
The real rate of undertreatment can be given as 3.2, 2.3, and 5.4%, respectively, for the blockers of the renin–angiotensin system, beta-adrenergic blockers, and MRAs.
With respect to the target dosages of these drugs, far fewer than one-third of the patients were on the target dosages suggested by the current guidelines: 29.3% for ACE inhibitors, 24.1% for ARBs, 17.5% for beta-blockers, and 30.5% for MRAs (Table 5). The reasons for not achieving the target dosages are also reported in Table 5. In about a third of the patients not achieving the target dosages, a drug up-titration was still ongoing, while for about another third of patients not achieving the target, a clear reason was not reported (28.8% for ACE inhibitors, 29.3% for ARBs, and 29.2% for beta-blockers). A higher rate (46.9%) of absence of clear reasons for not achieving the target dosage was reported for MRAs.
| At target, n (%) | Not at target, n (%) | Reason for not at target, n (%) | ||
|---|---|---|---|---|
| ACE-I (4710 pts) | 1380 (29.3) | 3330 (70.7) | 1123 (33.7) | Still in up-titration |
| 866 (26.0) | Symptomatic hypotension | |||
| 264 (7.9) | Worsening renal function | |||
| 85 (2.6) | Hyperkalaemia | |||
| 29 (0.9) | Cough | |||
| 5 (0.2) | Angioedema | |||
| 958 (28.8) | Other/unknown | |||
| ARBs (1500 pts) | 362 (24.1) | 1138 (75.9) | 369 (32.4) | Still in up-titration |
| 295 (25.9) | Symptomatic hypotension | |||
| 115 (10.1) | Worsening renal function | |||
| 25 (2.2) | Hyperkalaemia | |||
| 1 (0.1) | Angioedema | |||
| 333 (29.3) | Other/unknown | |||
| Beta-blockers (6468 pts) | 1130 (17.5) | 5338 (82.5) | 1871 (35.1) | Still in up-titration |
| 904 (16.9) | Symptomatic hypotension | |||
| 586 (11.0) | Bradyarrhythmia | |||
| 185 (3.5) | Worsening HF | |||
| 146 (2.7) | Bronchospasm | |||
| 56 (1.1) | Worsening PAD | |||
| 33 (0.6) | Sexual dysfunction | |||
| 1557 (29.2) | Other/unknown | |||
| MRAs (4226 pts) | 1290 (30.5) | 2936 (69.5) | ||
| 864 (29.4) | Still in up-titration | |||
| 350 (11.9) | Hyperkalaemia | |||
| 284 (9.7) | Worsening renal function | |||
| 60 (2.0) | Gynaecomastia | |||
| 1378 (46.9) | Other/unknown | |||
- a ACE-I, ACE inhibitor; HF, heart failure; MRAs, mineralocorticoid receptor blockers; PAD, peripheral artery disease.
Use of devices in ambulatory patients with chronic heart failure
Figure 2 describes the rate of implantation of ICDs and CRT in the ambulatory patients with chronic HF.
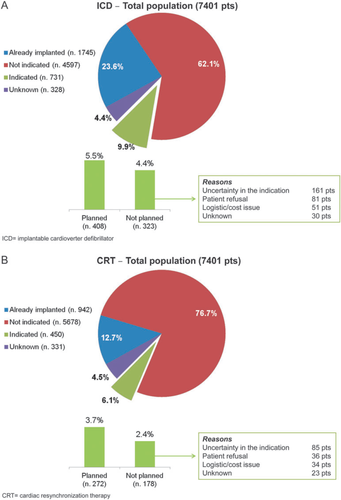
With respect to ICDs, in 62.1% of the cases the clinical characteristics that suggest, according to current guidelines, a device implantation with an ICD were not present (Figure 2A). Of the remaining cases, 23.6% of patients were already implanted with an ICD, 9.9% had the characteristics for the implantation but were not implanted, and for 4.4% there were no sufficient data to establish the indication. Of the 9.9% of patients with the indication but not implanted, in 5.5% the procedure was planned, while in the remaining 4.4% (44.2% of those with the indication) the implantation was not planned due to medical uncertainty of the indication, patient refusal, or logistical/cost issues.
As far as CRT is concerned (Figure 2B), 62.1% of the cases did not present the clinical characteristics that suggest, according to current guidelines, a CRT implantation. Of the remaining cases, 12.7% were already implanted with a CRT-P or CRT-D, 6.1% had the characteristics for the implantation but were not implanted, and for 4.5% there were insufficient data to establish the indication. Of the 6.1% of patients with the indication but not implanted, in 3.7% the procedure was planned, while in the remaining 2.4% (39.6% of those with the indication) the implantation was not planned due to medical uncertainty of the indication, patient refusal, or logistical/cost issues.
Discussion
Currently, reports of trials or registries concerning treatments of patients with HF, as well as in other conditions, quantitatively define the use of guideline-recommended ‘optimal therapy’, usually as the proportion of patients who received these treatments, but, specifically for drugs, regardless of dose. Current observations in patients with HF, for which therapy is particularly complex, show that a large proportion of the affected population fail to receive the recommended drugs and substantially less than half receive the ‘target doses’.2 However, reasons underlying this discrepancy between recommendations and actual clinical practice are not known. This ESC Long-Term Registry reflects ‘real-world’ management from a variety of hospitals, of all levels of complexity, and from all regions of Europe and Mediterranean countries. Detailed information has been obtained not only on patient characteristics and the use of procedures and treatments, but also on the reasons why recommended pharmacological or non-pharmacological treatments are not appropriately utilized in patients with HF. This information has generally not been available from previous administrative data sets, trials, or registries.
Characteristics of patients
Patients included in the ESC Long-Term Registry present with baseline characteristics, clinical history, and co-morbidities which largely overlap the observations of other European or US registries.1,2,17–21 Patients hospitalized for acute HF show a more severe clinical profile, as well as a higher rate of co-morbidities than patients with chronic HF. The substantial similarity of this population of patients with respect to previous reports allows the findings of this registry to be considered largely applicable also to other clinical contexts.
Treatments of hospitalized patients with acute heart failure
The treatment of patients hospitalized for acute HF remains largely opinion based, with little good evidence to guide therapy.6 Even in this context, the treatment algorithm suggested by the recent ESC guidelines6 recommends the administration of diuretics to all patients with congestion and, thereafter, different treatments according to the levels of SBP: specifically inotropes if SBP is < 85 mmHg; vasodilators (i.e. i.v. nitrates) if SBP is > 110 mmHg; and only diuretics with careful observation of the patients if SBP is between 85 and 110 mmHg.
Data from our registry show that > 80% of patients received a diuretic treatment, and that, despite the use of inotropes being more frequent, as expected, in patients with low SBP, a non-negligible proportion of patients received this treatment even with higher levels of SBP, and 6.8% of them even if SBP was > 110 mmHg. Similarly for i.v. nitrates, their prescription according to the levels of SBP does not appear adherent to the guideline suggestions.
Even considering the cut-off values of SBP suggested by previous guidelines,5 the general framework describing the use of inotropes and nitrates remains the same. Therefore, besides the fact that guidelines in this specific clinical context are not developed from evidence-based observations, the application in practice of these common-sense-based recommendations is not appropriate. This finding is similar to the observations of other registries.21–24
With respect to oral treatments, the rate of use of RAS blockers, beta-blockers, and MRAs significantly increases at discharge after the index admission. This fact documents furthermore that the admission for HF is, for a relevant number of patients, a concrete opportunity to optimize their background therapy. This consideration is not valid for device implantation, which is not significantly modified during or after hospital admission.
Treatments of ambulatory patients with chronic heart failure
The ESC-HF Long-Term Registry provides a clear picture of the rate of use of guideline- recommended, evidence-based treatments; and detailed data on the proportion of patients in whom the target suggested dose was reached as well as the adherence to recommendations on device implantation are available. However, unlike other registries or trials, it also exposes the reasons for non-adherence to the guidelines recommendations.5,6,25
As was also demonstrated in the ESC-HF Pilot Survey,14 the rate of use of renin–angiotensin–aldosterone system blockers (ACE inhibitors, ARBs, and MRAs) and beta-adrenergic blockers is satisfactory. This registry can expand this observation showing that, among the relatively small proportion of patients not receiving ACE inhibitors, ARBs, or beta adrenergic blockers, the rate of real undertreatment is even smaller, a documented contraindication or intolerance being clearly reported in the majority of untreated patients. A different consideration must be made for MRAs, for which the recommendation for treatment, during the period of data collection, was still limited by previous guidelines5 to the most severe patients (NYHA class III–IV). Considering this context, the percentage of patients for whom there is no documented explanation for non-prescription was 5.4%.
Although the rate of use of treatments with a recommendation of type 1A was confirmed to be satisfactory, the number of patients treated with appropriate doses was very far from optimal, being no more than one-third. This is a common finding of most registries or trials on HF,14,26,27 confirming the gap between the dosages used in randomized controlled trials, performed in selected populations carefully monitored over time, and those provided by observational research reflecting routine clinical practice. This huge ‘underdosing’ might be related to physician inexperience, undesired effects, co-morbidities determining polypharmacy, and then limiting the up-titration generally performed in the original trials from which the targets were derived. If the recommended target doses in the current guidelines are appropriate, we might infer that most patients are currently not treated adequately. If we take the opposite point of view, the data collected from patients enrolled in the ESC-HF Long-Term Registry might suggest that the range of doses currently used in clinical practice appropriately represents patient needs and drug tolerability in current clinical practice. The evaluation of the reasons for non-adherence to recommended dosages shows that, in the majority of cases, in whom target doses were not achieved, reasonable clinical motivations related mostly to drug up-titration still ongoing or intolerance were reported, greatly reducing the number of patients for whom no documented explanations for low dosages have been reported.
Concerning the rate of implantation of devices (a CRT and/or ICD), those patients with a clinical profile theoretically corresponding to the indication for device implantation were identified. Of the 450 patients who qualified for CRT implantation, in 272 the procedure was planned, while in 178 (40%) patients doctor uncertainties or patient refusal were reported. Similar figures have been described for ICD implantation: of 731 patients indicated for ICD, in 408 the implantation was planned, while for 323 (44%) the procedure was not planned. As expected, logistical or cost issues were also reported as the cause of non-implantation of CRT or ICD.
This is certainly a gap between recommendations and actual clinical practice that should be considered, but underuse of device implantation seems to be less than described in previous observations.14,16
Limitations
Some important limitations of our registry must be acknowledged.
First, criteria for HF diagnosis were discussed during the investigator meetings, and the Guidelines of reference were commented on and disseminated to all investigators. However, the diagnoses were made by the investigators according to their clinical judgement and not validated centrally. Secondly, although we tried to balance the methodological need for consecutiveness of enrolment with the practical feasibility, thereby decreasing the workload for centres by limiting recruitment to 1 day per week for 12 months, we cannot prove the consecutiveness of patient enrolment. Thirdly, representativeness is often recognized as a limitation in observational studies. To lessen this problem, the centres were selected in proportion to the size of the population of the participating countries, taking into account the different technological levels of the cardiology centres invited to participate. Fourthly, the patients were all enrolled in cardiology wards and clinics, and they generally did not include those presenting at the Emergency Department, and/or admitted to other hospital facilities. Accordingly, the population reported herein does not represent the whole gamut of patients with HF.
Conclusions
Evidence-based clinical practice guidelines have been developed for the treatment of patients with HF;5–7,28 however, substantial gaps and variations currently exist in the quality of care provided to patients with HF. The data collected in this registry show that, while in patients with acute HF a large heterogeneity of treatments exists, mainly due to the absence of clear evidence-based recommendations, the pharmacological treatment of patients with chronic HF can be considered acceptably adherent to the recommendations of current guidelines. This is because the real rate of undertreatment or underdosage of drugs is shown to be limited when the reasons for non-adherence are appropriately taken into account. With respect to device implantation, the gap between guidelines and practice seems to be greater, probably due to different local medical practice but also to differences in healthcare systems.
Observations regarding the real possibility to adhere fully to current guidelines in daily clinical practice should be taken into account when clinical practice guidelines are written, also because guideline recommendations are increasingly perceived as mandatory for all patients, with potential legal consequences if they are abrogated.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Funding
The Survey was funded by the ESC. Each participating national cardiology society was given a grant of €10 000 to help with the organizational needs of national network implementation.
The following companies support the EURObservational Research Programme: Gold-level support: Abbot Vascular, Bayer Pharma, BMS/Pfizer, Boehringer Ingelheim International, Daiichi Sankyo Europe, Menarini International, Novartis Pharma, Laboratoires Servier. Silver-level support: Amgen. Bronze-level support: Boston Scientific International, MSD/Merck & Co, Sanofi-Aventis Group.
Acknowledgements
The Executive Committee of the study had full access to the data and takes complete responsibility for the integrity and the accuracy of the data analysis. The authors would like to thank Renato Urso for the statistical help and Barbara Bartolomei Mecatti for editorial assistance.
Conflict of interest:
None declared.
Appendix 1. Committees and Investigators
Executive Committee: A.P. Maggioni, S.D. Anker, U. Dahlström, G. Filippatos, P. Ponikowski, L. Tavazzi, F. Zannad
Steering Committee: O. Amir, O. Chioncel, M. Crespo-Leiro, J. Drozdz, A. Erglis, E. Fazlibegovic, C. Fonseca, F. Fruhwald, P. Gatzov, E. Goncalvesova, M. Hassanein, J. Hradec, A. Kavoliuniene, M. Lainscak, D. Logeart, B. Merkely, M. Metra, H. Persson, P. Seferovic, A. Temizhan, D. Tousoulis
EORP team: M. Andarala, E. Fiorucci, E. Folkesson, M. Glemot, G. Gracia, M. Konte, C. Laroche, PA. McNeill, V. Missiamenou, C. Taylor
Investigators
AUSTRIA Graz: F. Fruhwald, A. Eberl, S. Heller, T. Dolze, V. Platzer, K. Ablasser; Braunau: J. Auer; Innsbruck: G. Poelzl; Sankt Poelten: D. Moertl; Vienna: A. Podczeck-Schweighofer, S. Reiter. BOSNIA HERZEGOVINA Mostar: D. Pravdic, E. Fazlibegovic, A. Muslibegovic. BULGARIA Sofia: K. Vitlianova, T. Katova, K. Kotirkov, I. Petrov, T. Velikov, T. Kurteva, P. Gatzov; Vidin: D. Kamenova, M. Antova; Pleven: S. Tisheva; Varna: V. Sirakova. CZECH REPUBLIC Brno: J. Krejci J. Spinar, M. Mikolaskova; Prague: J. Krupicka, F. Malek, M. Hegarova; Hradec Kralove: R. Pudil; Znojmo: Z. Monhart, Olomouc: M. Lazarova; EGYPT Cairo: A. Reda, T. Khairy, N. Farag, A. Saleh, K. Said, M. Abdel Hamid, S. Halawa; Giza Cairo: B. Ibrahim, R. Hanna; Alexandria: M. Hassanein, M. Sobhy, F. El Messiry, A.H. El Shazly, Y. Elrakshy; Zagazig: W. Aboleineen, M. Gouda; Assiut: A. Youssef; Port Said: A. Elbahry; Benha: A. Abdel; Damanhour: H. Ebeid; Ismailya: G. Nasr; Tanta: H. Sobhy,M. Ashmawy. FRANCE Paris: C. Lefol, R. Isnard, A. Hagege, S. Thevenin, G. Jondeau, D. Logeart; Senlis: R. Codjia; Toulouse: M. Galinier; Rouen: F. Bauer, Bron: F. Delahaye; Lille: N. Lamblin, M. Fertin, P. de Groote; Poitiers: D. Coisne, V. Le Marcis, B. Lequeux, J.-F. Ly; Rennes: P. Lotton, E. Donal, V. Le Moal, A. Basquin, A. Reynaud, C. Ridard, C. Thebault, N. Behar, S. Mascle; Besançon: F. Schiele, M.-F. Seronde, M. Morel, N. Meneveau, P. Luporsi, Y. Bernard, M. Pillot, F. Briand; Creteil: T. Damy, Clermont-Ferrand: J.-C. Eicher. GREECE Athens: C. Avgeropoulou, T. Panagiotis, D. Tousoulis, I. Kotrogiannis, C. Stefanadis, C. Chrysohoou, G. Tsitsinakis, T. Dimitroula, A. Karavidas, V. Matzaraki, J. Terrovitis, C. Kapelios, T. Sfakianaki, G. Filippatos, J.T. Parissis, K. Ntai; Volos: A. Papadopoulou, H. Tziourganou, A. Douras, K. Ntertsas, T. Karotsaki, N. Nikoloulis, T. Tsaknakis; Heraklion: A. Patrianakos, E. Nyktari, P. Vardas. HUNGARY Budapest: P. Soos, N. Nyolczas, A. Csaba Nagy; Pecs: R. Halmosi; Esztergom: F. Nemeth, G. Amon, J. Dinnyes. ISRAEL Hadera: A. Vazan Fuhrmann, A. Shotan, I. Alony, J. Elber. ITALY Modena: A. Olaru, C. Valenti, L. Reggianini, M. G. Modena, S. Copelli, C. Leuzzi; Brescia: L. Dei Cas, V. Carubelli, C. Lombardi, M. Bulgari, E. Tanghetti, F. Quinzani, M. Metra, R. Rovetta; Roma: B. Brasolin, F. Fedele, D. Sergi, F. Romeo, I. Scarfò, M. Vellini; Trieste: E. Fabris, M. Cinquetti, D. Stolfo, G. Sinagra, M. Merlo; Ferrara: A. Fucili, R. Ferrari; Verona: C. Vassanelli, F. Torelli, G. Marchese, L. Zanolla, M. Cicoira; San Bonifacio: M. Anselmi; Novara: C. Piccinino, C. Sartori, M.V. Di Ruocco, P.N. Marino; Lumezzane: A. Giordano, S. Brognoli, E. Zanelli; Cortona: F. Cosmi; Monserrato: C. Cadeddu, S. Bandino, G. Mercuro, M. Deidda; Foggia: G. Salvemini, M. Correale, M. Di Biase, S. Musio, T. Passero; Milano: G. Italiano, P. Agostoni, E. Salvioni; Cotignola: G. Schiavina, A. Squeri, S. Bosi, A. Barbieri; Atri: E. Lemme, M. Penco, S. Marcon, S. Romano, M. Di Mauro; Passirana Di Rho: A. Frisinghelli, M. Veniani; Palermo: P. Pieri, S. Novo; Udine: A. Proclemer, C. Fresco, D. Miani, J. Artico; Torino: F. Gaita, S. Bergerone; Cremona: S. Pirelli, G. Di Tano; Pavia: S. Cattaneo, A. Gualco, C. Opasich, S.G. Priori; Sassuolo: E. Iori, E. Leci, F. Melandri. LATVIA Jelgava: N. Voronina; Riga: A. Erglis, A. Kalnins. LITHUANIA Kaunas: A. Kavoliuniene, E. Rumbinaite, E. Kazakauskaite, I. Petraskiene, R. Karaliute, V. Smalinskas, V. Tamakauskas; Marijampole: D. Petraskiene, R. Brazyte-Ramanauskiene, V. Vysniauskas. POLAND Poznan: A. Nowicka, J. Grabia, R. Dankowski, A. Szyszka, K. Szymanowska, E. Straburzynska-Migaj, M. Kaluzna-Oleksy, S. Grajek; Chelmza: P. Kasztelowicz, T. Orzel; Sieradz: G. Bednarczyk, P. Ruszkowski; Warszawa: J. Klimkiewicz, A. Majzner, A. Matusiak, E. Bylicka, E. Kwiatkowska, K. Kwiatkowska, M. Ochnio, T. Krzyzewska, A. Tarnowska, K. Komuda, M. Sobieszczanska-Malek, P. Leszek, T. Rywik, J. Wisniewska, K. Kozar-Kaminska, M. Piotrowska, T. Zielinski, A. Mamcarz, M. Welnicki, A. Folga, A. Kaplon-Cieslicka, G. Opolski, M. Marchel, P. Balsam, A. Michalek, A. Pawlak, K. Byczkowska, O. Mozenska, K. Gil, R.J. Gil, A. Skrobowski, B. Uzieblo-Zyczkowska, K. Piotrowicz, P. Krzesinski, A. Stanczyk, M. Ciurzynski, P. Bienias, P. Pruszczyk; Biala: Z. Juszczyk, P. Switala, S. Stankala; Zabrze: L. Polonski, P. Rozentryt; Czestochowa: J. Gabryel, M. Lazorko-Piega, P. Kardaszewicz; Pruszkow: I. Poprawa, E. Komorowska, A. Lauko-Rachocka, A.-F. Abdulkarim, A. Dudzik-Plocica, A. Zolbach, D. Sajnaga, K. Komorowska, K. Baczynska, M. Samcik, L. Wolniewicz; Kielce: B. Wozakowska-Kaplon, B. Sosnowska-Pasiarska, R. Bartkowiak; Starachowice: A. Fiega, A. Serwicka, A. Szymczyk, G. Fitas; Strzegom: D. Zysko; Lodz: A. Poliwczak, M. Broncel, A. Bala, J.D. Kasprzak, L. Jankowski, M. Rudzinska, P. Zycinski, A. Retwinski, I. Pietka, J. Drozdz, K. Wojtczak Soska; Wroclaw: E. Jankowska, P. Ponikowski; Przeworsk: A. Cichy, A. Kostka, L. Kaminski; Lublin: J. Weglarz, A. Bodys, P. Flis; Zamosc: A. Kleinrok, G. Prokop-Lewicka; Gdansk: K. Mosakowska, A. Rynkiewicz, J. Bellwon, A. Dabrowska-Kugacka, E. Lewicka, G. Raczak; Szczecin: E. Skorek, S. Szabowski, W. Krysiak; Bydgoszcz: J. Pietrzak, W. Gilewski, W. Sinkiewicz; Krakow: A. Furman, J. Zalewski, J. Nessler, K. Bury, A. Grzegorzko, M. Zabojszcz, E. Mirek-Bryniarska; Zabrze: J. Klys, K. Przybylska, M. Szulik, Z. Kalarus, A. Kuczaj, E. Nowalany-Kozielska; Kluczbork: A. Krzeminski. PORTUGAL Carnaxide: A. Ventosa, C. Tavares Aguiar; Porto: B. Moura, J.S. Cardoso; Faro: I. De Jesus, J. Chin, R. Faria, S. Pereira; Lisboa: G. Lima, T. Oliveira Guimarães, A.R. Francisco, D. Brito, R. Placido, S. Martins, C. Fonseca, F. Marques, I. Araujo, M. Proenca, R. Cardiga; Guilhufe-Penafiel: A. Andrade, A. Castro, A. Pereira, C. Queirós, P. Silva, R. Santos, C. Lourenço, N. Moreno; Guimaraes: F. Almeida, I. Quelhas; Vila Real: A. Baptista, A. Ferreira, P. Magalhaes, C. Ferreira, I. Moreira; Santarem: D. Durao, D. Severino, G. Ferreira da Silva, L. Marta, M. Peres. ROMANIA Bucharest: C. Stanescu, A. Dan, G-A. Dan, I. Daha, R. Popescu, D. Vinereanu, C-J. Sinescu, C. Macarie; Brasov: M. Radoi; Galati: E. Nechita; Timisoara: R. Christodorescu; Iasi: M. Datcu; Constanta: E. Craiu. SERBIA Belgrade: P. Otasevic, A.D. Ristic, D. Simeunovic, P. Seferovic, B. Pencic, A. Andric, B. Stojcevski, E. Lebedinski, J. Suzic Lazic, V. Celic, M. Krotin, S. Radovanovic; Kragujevac: V. Iric-Cupic, S. Milanov, G. Davidovic, M. Jovic; Sremska Kamenica: A. Radin, B. Mihajlovic, J. Stojiljkovic, M. Cankovic, N. Cemerlic Adic, S. Dodic, S. Kecojevic, S. Stojsic; Niska Banja: D. Petrovic, M. Deljanin Ilic, S. Ilic, V. Stoickov; Nis: S. Antic, D. Stanojevic, M. Pavlovic, V. Atanaskovic, V. Mitic, SLOVAKIA Martin: F. Kovar; Bratislava: E. Goncalvesova, J. Murin, M. Pernicky; Banovce Nad Bebravou: A. Klabnik; Presov: J. Kmec SLOVENIA Murska Sobota: D. Rajtman, D. Kovac, S. Horvat; Maribor: B. Krunic, M. Bombek, I. Krajnc, R. Losic, T. Glavic; Izola: J. Komel, N. Cernic Suligoj, T. Ravnikar; Ptuj: M. Letonja, V. Cencic; Brezice: M. Savnik Iskra, M. Strasek; Trbovlje: B. Leskovar, B. Drnovsek, J. Klen; Slovenj Gradec: A. Marolt, M. Kladnik, C. Slemenik Pusnik, M. Pusnik Vrckovnik; Ljubljana: B. Jug, Z. Fras; Sempeter Pri Novi Gorici: A. Bartolic, C. Melihen-Bartolic, M. Valentincic, R. Winkler. SPAIN La Coruña: P. Blanco-Canosa, Z. Grille-Cancela, M. Crespo-Leiro, M.J. Paniagua-Martin, R. Marzoa-Rivas, E. Barge-Caballero; Marbella: F. Torres Calvo, R. Bravo Marques, F. Epelde Gonzalo; Valladolid: A. Recio Platero, L. de la Fuente Galan, J. Lopez Diaz, L. Renier Goncalvez Ramirez, H. Cubero Gallego, J.F. Sliwiski Herrera; Tortosa: D. Bierge Valero; Albacete: F.M. Salmeron, J.C. Gallego Page, M. Jose Fernandez; Manacor: A. Sahuquillo, B. Garcia de la Villa; Madrid: A. Castro Conde, A.M. Iniesta Manjavacas, O. Salvador Montanes, R. Dalmau Gonzalez-Gallarza, S. Ofelia Rosillo, A. Araujo, A. Briceno, L. Alonso-Pulpon, M. Cobo, T. Soria, M. Gomez-Bueno, P. Garcia-Pavia, A. Gonzalez-Segovia, I. Sayago, J. Segovia Cubero, J. F. Delgado Jiménez, M. Angel Gomez Sanchez, P. Escribano Subias, E. Barrios Garrido-Lestache, M.J. Ruiz Cano, M. Vicente Hernandez; Granada: M. Puga-Martinez, E. Lopez-Moreno, J.L. Serrano-Martinez, M. Fernandez-Alvarez, R. Rivera-Lopez, S. Lopez-Fernandez, V. Alcade-Martinez; Vigo: M. Sanmartin; Valencia: A. Pellicer-Cabo, D. Garcia-Escriva, F. Ridocci-Soriano, J. Perez-Silvestre, L. Facila-Rubio, P. Garcia-Gonzalez, I.J. Sanchez-Lazaro, L. Almenar-Bonet; Malaga: J.M. Garcia Pinilla; Barakaldo: J. Andres; Barcelona: A. Bayes Genis, E. Roig, S. Mirabet, A. Mendez, L. Garcia-Cosio, A. Garay, G. Muntane, J. Gonzalez-Costello, V. Leon; Oviedo: B. Diaz Molina; Santa Cruz De Tenerife: I. Famara Hernandez, A. Lara Padron, I. Laynez Cerdena; San Juan De Alicante: I. Mateo, J. Quiles, V. Bertomeu, A. ElAmrani, J. Angel Rodrigez-Ortega, R. Martinez-Abellan, R. Valero, Sevilla: C. Fernandez-Vivancos; Zaragoza: M. Sanz Julve; Murcia: M.T. Perez-Martinez, D.A. Pascual-Figal, M.D. Martinez Martinez-Espejo, M. Rosario Gracia-Rodenas, F. Pastor-Perez. SWEDEN: Stockholm: M. Melin, E. Hägglund; Lindesberg: A. Stenberg, I.-M. Lindahl; Varberg: B. Asserlund, L. Olsson; Linköping: U. Dahlström, M. Afzelius; Jönköping: P. Karlström, L. Tengvall; Kristianstad: P-A. Wiklund, B. Olsson. TURKEY Ankara: A. Temizhan, S. Kalayci, M. Bozkurt; Antakya/Hatay: M. Taraktas; Eskisehir: Y. Cavusoglu; Sivas: H. Gunes, M. Birhan Yilmaz; Istanbul: B. Demir; Kilis: E. Gencer.




