Trends in long-term mechanical circulatory support for advanced heart failure in the UK
Abstract
Aims
Heart transplantation (HTx) is limited by the scarcity of suitable donor hearts. Consequently, more patients with advanced heart failure require a ventricular assist device (VAD). We report UK activity, trends, and outcome for long-term VAD support as a bridging therapy to HTx.
Methods and results
Patients were grouped into three eras: E1, February 2004–March 2006; E2, April 2006–March 2009; and E3, April 2009–March 2011. Exclusions were patients who received isolated short-term support or extracorporeal membrane oxygenation without prior or subsequent long-term VAD support. A total of 247 patients received VAD support; 202 left ventricular (LVAD) support alone and 45 both left and right ventricular support. Activity increased over time, from 36 patients implanted in E1 to 123 in E3. Overall, 46 patients received a first-generation device, 80 a second-generation device, and 121 a third-generation device. Use of third-generation devices increased from <6% in E1 to 78% in E3. Median duration of LVAD support increased from 141 days in E1 to 578 days in E3 (P < 0.001). Overall survival to 1 year after LVAD implant rose from 58.3% [95% confidence interval (CI) 40.7–72.4%] in E1 to 72.5% (95% CI 63.3–79.8%) in E3 (P = 0.21), and improved significantly with device generation; at 1 year, 50% of patients with first-generation devices were alive compared with 68.1% and 76.9% of patients with second- and third-generation devices, respectively (P = 0.002). These differences remained after risk adjustment. HTx following LVAD implant reduced over time (P < 0.001).
Conclusion
VAD activity and duration of support have increased. There has been a shift from first- and second- to third-generation devices, and an associated improvement in survival.
See page 1185 for the editorial comment on this article (doi: 10.1093/eurjhf/hft137)
Introduction
Despite evidence-based medical therapy, heart failure (HF) is frequently a progressive condition, and advanced HF is associated with a poor prognosis and quality of life. Heart transplantation (HTx) is a highly effective treatment for a selected subgroup of these patients, but it is limited by the scarcity of suitable donor hearts, resulting in increasing waiting times before transplantation.1 Consequently, many patients now need to be bridged to transplantation using mechanical circulatory support.2–4
The technology available for circulatory support has developed rapidly during the last decade, with a progression from first- to second- and, now, third-generation devices. For patients in advanced HF, survival on support has been found to exceed that on medical therapy;4,5 but, as yet, such therapy has not been deemed cost-effective by the criteria established by the UK National Health Service (NHS), and the longer term outcome of such treatment remains to be established. While the use of LV assist devices (LVADs) has been restricted to bridging patients to transplantation in the UK,6 limited heart transplant activity has resulted in patients receiving longer periods of mechanical support.
In the USA, trends in LVAD activity and outcome have been documented in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS),7 but this only includes devices implanted after final approval by the US Food and Drug Administration (FDA) and so excludes newer devices.8 LVAD registries have been established in Europe, but are voluntary and so are not comprehensive. In the UK, nearly all LVAD implants have been performed in NHS hospitals as part of the bridge-to-transplant programme, and the UK Cardiothoracic Transplant Audit (UKCTA) has established a Mechanical Circulatory Support Registry with mandatory data reporting, thus providing a unique and comprehensive national database of mechanical circulatory support.
Here we report trends in UK activity and outcome after the implantation of LVADs intended to provide long-term outpatient therapy for advanced HF recorded in the UKCTA database.
Methods
The UKCTA is an ongoing prospective cohort study involving all UK HTx centres (see Appendix 2), which has collected data on all patients listed for HTx and patients who received allografts since April 1995. Limited data on VAD activity and outcome (demographic data and follow-up data for survival, explant, and transplant) have been collected since 2002. Centres are responsible for notifying the National Health Service Blood and Transplant (NHSBT) after each VAD implantation. In 2006, a more comprehensive database was proposed for the purposes of audit and research. The UKCTA project group designed the database and proposed the variables to be recorded. The final data set was then agreed with the UK VAD Forum and the UKCTA Steering Group. Since November 2009, centres have submitted data prospectively. For VAD activity between 2004 and November 2009, centres were funded to enter data retrospectively.
Data collection
Data from patients receiving mechanical circulatory support have been collected at implant, 7 days post-implant, 1 and 3 months post-implant, and thereafter every 3 months until explant, transplant, or death. HTx audit data were collected at listing, at transplant, at 3 months, and annually thereafter until the patient's death. The data are processed by NHSBT and then submitted on a monthly basis to the UKCTA. A linkage protocol allows data from the VAD and HTx registries to be combined. Ninety-five per cent of all VAD implants prior to November 2009 have been recorded within this database. The data are checked and validated by NHSBT and the UKCTA in an ongoing data validation exercise including randomized checking of original patient records. Written informed consent is sought when listing the patient for transplantation and when a VAD is implanted. In the UK audit, projects do not require separate research ethics committee approval.
Study population
All adults (age 16 years and over) who received an implantable LVAD intended for long-term outpatient therapy between February 2004 and March 2011 were included in the analysis. Exclusions were patients who received short-term inpatient support with a device that was not intended to allow the patient to be discharged from hospital, such as the Levitronix/Thoratec CentriMag, or ventriculoarterial extracorporeal membrane oxygenation (ECMO) without prior or subsequent long-term VAD support. The database was closed for analysis on 13 February 2012.
Patients were grouped into three eras according to the date of the first implant: Era 1, February 2004–March 2006; Era 2, April 2006–March 2009; and Era 3, April 2009–March 2011.
Implantable LVADs were categorized as: first generation (pulsatile, volume displacement), second generation (axial flow), and third generation (bearing-less centrifugal flow).
Patients had a VAD implanted for LV failure (LVAD) or, for the failure of both ventricles, biventricular (BiVAD) support.
Patients were grouped using INTERMACS classification: 1/1A, critical cardiogenic shock; 2/2A, progressive decline; 3/3A, stable but inotrope dependent; 4/4A, recurrent advanced heart failure; 5/5A, exertion intolerant; 6/6A, exertion limited; 7, advanced NYHA class 3. In each class, category A (e.g. 1A, 2A, etc.) is with recurrent ventricular tachycardia/ventricular fibrillation (VT/VF).9
Statistical methods
Continuous variables were summarized using the mean and standard deviation or median and interquartile range as appropriate, and categorical variables were reported as a number and percentage. Groups were compared using the t-test, Wilcoxon rank sum, χ2, or Fisher's exact tests as appropriate.
Patient survival and duration of VAD support was estimated by the Kaplan–Meier method and compared using the log rank test. Patient survival was defined as the time from first implant to death. For patients alive at the time of analysis, survival was censored at last known follow-up. The duration of support was defined as the time from first implant to VAD explant, transplant, or death. For patients alive on VAD support at the time of analysis, the duration of support was censored at last known follow-up. If the VAD was replaced, support on the replacement device was included in the overall duration of support. The cumulative incidence of the outcome on support (explant without a transplant, HTx, or death) and of different causes of recipient death was estimated in the presence of competing risks.
Patient survival from first implant to death, adjusted for generation of VAD, age, gender, explant without a transplant, and HTx after VAD support, was compared across eras using Cox proportional hazards regression. As VAD explant and transplant occurred during follow-up, they were modelled as time-dependent covariates, i.e. they were set to ‘no’ at time 0 (LVAD implant) and changed to ‘yes’ at time t when the explant or HTx took place. To capture the changing risk of death post-HTx, the hazard was estimated separately for days 0–7, 8–30, 31–90, and days 90+ after HTx.
Analyses were carried out using Stata version 11.2
Results
Long-term ventricular assist device support
A total of 247 patients received long-term VAD implants for LV failure during the study period, 36 in Era 1, 88 in Era 2, and 123 in Era 3. Fourteen patients had received prior short term support and five patients received replacement LVADs, three due to device malfunction. Overall, 202 patients received LVAD support alone and 45 received BiVAD support. No patient received long-term right ventricular assist device (RVAD) support alone. Fifteen patients were implanted with the intention to bridge to a decision about suitability for transplantation and 232 patients were implanted with the intention of bridging to transplant. Patient characteristics are shown in Table 1. The majority of patients (157, 63.6%) had a diagnosis of non-ischaemic dilated cardiomyopathy and were in INTERMACS category 2/2A (114, 46.2%). The proportion of patients in INTERMACS category 1/1A has decreased over time, while the proportion in category 3/3A has increased.
| Variable | Overall, n (%) | Era 1, n (%) | Era 2, n (%) | Era 3, n (%) | P-value |
|---|---|---|---|---|---|
| Patients within the study period | 247 | 36 | 88 | 123 | |
| Demographics | |||||
| Age in years, median (IQR) | 47.0 (35.2–55.9) | 46.8 (29.5–56.8) | 43.5 (29.6–54.7) | 49.2 (39.0–56.3) | 0.061 |
| Male gender | 197 (80.0) | 29 (80.6) | 69 (78.4) | 99 (80.5) | |
| Co-morbidities | |||||
| Diabetic | 33 (13.8) | 3 (9.1) | 10 (11.5) | 20 (16.8) | 0.39 |
| Smoking status | |||||
| Non-smoker or ≤5 per day | 123 (60.0) | 19 (67.9) | 47 (63.5) | 57 (55.3) | 0.58 |
| Ex-smoker >6 months | 61 (29.8) | 6 (21.4) | 19 (25.7) | 36 (35.0) | |
| Still smoking (>5/day in last 6 months) | 21 (10.2) | 3 (10.7) | 8 (10.8) | 10 (9.7) | |
| COPD | 2 (0.9) | 0 (0) | 0 (0) | 2 (1.7) | 0.36 |
| Previous vascular disease | 3 (1.3) | 1 (3.2) | 2 (2.4) | 0 (0) | 0.20 |
| Neurovascular disease | |||||
| Previous ischaemic stroke | 10 (5.2) | 1 (3.9) | 3 (4.4) | 6 (6.3) | 0.21 |
| Previous transient ischaemic attack | 11 (5.8) | 4 (15.4) | 2 (2.9) | 5 (5.2) | |
| None | 170 (89.0) | 21 (80.8) | 64 (92.8) | 85 (88.5) | |
| Hypertension | 21 (9.8) | 3 (9.7) | 4 (5.0) | 14 (13.5) | 0.16 |
| Ascites | 24 (11.1) | 1 (3.7) | 9 (11.5) | 14 (12.6) | 0.41 |
| HLA sensitized | 26 (17.0) | 2 (10.0) | 9 (15.5) | 15 (20.0) | 0.53 |
| Previous myocardial infarction | 64 (28.1) | 11 (33.3) | 15 (19.0) | 38 (32.8) | 0.08 |
| No. of previous sternotomiesa | |||||
| 0 | 159 (82.4) | 23 (82.1) | 46 (79.3) | 90 (84.1) | 0.38 |
| 1 | 29 (15.0) | 3 (10.7) | 10 (17.2) | 16 (15.0) | |
| 2 | 5 (2.6) | 2 (7.1) | 2 (3.5) | 1 (0.9) | |
| No. of previous thoracotomiesa | |||||
| 0 | 174 (98.3) | 26 (100) | 55 (98.2) | 93 (97.8) | 0.85 |
| 1 | 2 (1.1) | 0 (0) | 1 (1.8) | 1 (1.1) | |
| 2 | 1(0.6) | 0 (0) | 0 (0) | 1 (1.1) | |
| Previous IABP | 83 (35.9) | 19 (59.4) | 29 (35.4) | 35 (29.9) | 0.021 |
| Previous ECMO | 6 (2.6) | 0 (0) | 3 (3.6) | 3 (2.6) | 0.55 |
| Diagnosis at implant | |||||
| Ischaemic heart disease | 66 (26.7) | 11 (30.6) | 10 (11.4) | 45 (36.6) | 0.001 |
| Non-ischaemic dilated cardiomyopathy | 157 (63.6) | 23 (63.9) | 67 (76.1) | 67 (54.5) | |
| Restrictive cardiomyopathy | 8 (3.2) | 1 (2.8) | 5 (5.7) | 2 (1.6) | |
| Hypertrophic cardiomyopathy | 7 (2.8) | 0 (0) | 2 (2.3) | 5 (4.1) | |
| Congenital heart disease | 3 (1.2) | 0 (0) | 0 (0) | 3 (2.4) | |
| Valvular heart disease | 1 (0.4) | 0 (0) | 1 (1.1) | 0 (0) | |
| Other | 5 (1.6) | 1 (2.8) | 3 (3.4) | 1 (0.8) | |
| INTERMACS profile at first implant | |||||
| Prior short-term support | 14 (5.7) | 3 (8.3) | 8 (9.1) | 3 (2.4) | 0.016 |
| INTERMACS 1/1A | 37 (15.0) | 8 (22.2) | 11 (12.5) | 18 (14.6) | |
| INTERMACS 2/2A | 114 (46.2) | 16 (44.4) | 47 (53.4) | 51 (41.5) | |
| INTERMACS 3/3A | 47 (19.0) | 2 (5.6) | 11 (12.5) | 34 (27.6) | |
| INTERMACS 4/4A/5/6/7 | 35 (14.2) | 7 (19.4) | 11 (12.5) | 17 (13.8) |
- a COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; HLA, human leucocyte antigen; IABP, intra-aortic balloon pump; IQR, interquartile range.
- a Sternotomy and thoracotomy data were missing in 21% and 28% of records, respectively.
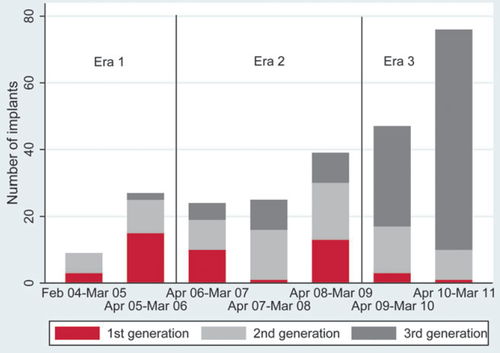
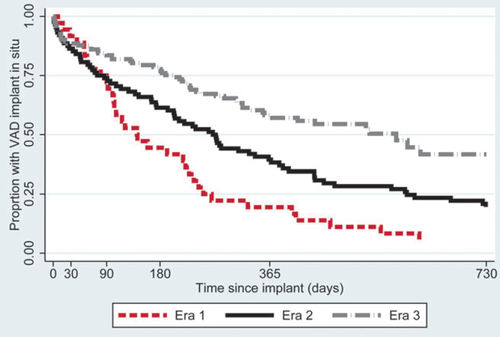
The LVADs used are shown in Table 2; 46 patients received a first-, 80 a second-, and 121 a third-generation device. Device use has changed over time: the number of first-generation devices has decreased and third-generation devices have become more common (Figure 1). In Era 1, the median duration of VAD support was 141 days (interquartile range 80–253 days), compared with 269 days (interquartile range 74–596 days) in Era 2 and 578 days (lower quartile 204 days) in Era 3 (Figure 2, P<0.001).
| Manufacturer | Device | Era 1, n | Era 2, n | Era 3, n | Total, n | % |
|---|---|---|---|---|---|---|
| First generation | ||||||
| Berlin Heart | EXCOR | 1 | 15 | 1 | 17 | 6.9 |
| Thoratec | PVAD | 9 | 3 | 1 | 13 | 5.3 |
| Thoratec | IVAD | 3 | 4 | 2 | 9 | 3.6 |
| Thoratec | HeartMate XVE | 5 | 2 | 0 | 7 | 2.8 |
| Total first generation | 18 | 24 | 4 | 46 | 18.6 | |
| Second generation | ||||||
| Thoratec | HeartMate II | 2 | 39 | 23 | 64 | 25.9 |
| Jarvik Heart | Jarvik 2000 | 12 | 1 | 0 | 13 | 5.3 |
| MicroMed | DeBakey | 2 | 1 | 0 | 3 | 1.2 |
| Total second generation | 16 | 41 | 23 | 80 | 32.4 | |
| Third generation | ||||||
| HeartWare | HVAD | 0 | 3 | 91 | 94 | 38.1 |
| Ventracor | VentrAssist | 2 | 20 | 5 | 27 | 10.9 |
| Total third generation | 2 | 23 | 96 | 121 | 49.0 | |
| Total all devices | 36 | 88 | 123 | 247 | 100 |
Patient survival
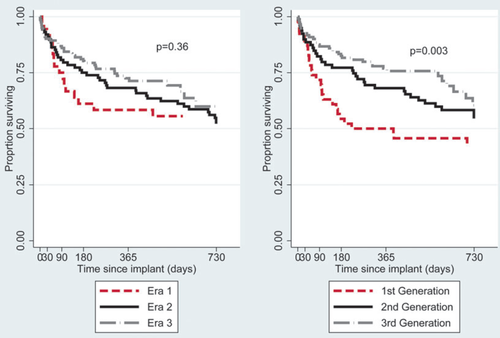
Patient survival to 2 years after implant is shown in Figure 3. Survival, unadjusted for patient risk, transplantation, or explant, has not changed significantly by era (P = 0.36), but has improved with device generation (P = 0.003). At 1 year, 50.0% of patients receiving a first-generation device were alive [95% confidence interval (CI) 34.9–63.3%] compared with 68.1% of patients receiving a second-generation device (95% CI 56.5–77.2%) and 76.9% of patients receiving a third-generation device (95% CI 68.0–83.6%). The corresponding survival estimates at 2 years were 43.4% (95% CI 28.9–57.0%), 54.5% (95% CI 42.3–65.2%), and 60.4% (95% CI 46.4–71.8%) for the first-, second-, and third-generation devices respectively.
Outcome of support
The outcome of LVAD support, i.e. explant, transplant, or death, is shown in Figure 4. At the time of analysis, in 30 cases the LVADs had been explanted, 63 patients had received a transplant, and 80 died on support. The remaining 74 patients were alive on support. The number of patients receiving a transplant after their VAD implant has reduced significantly over time, with many fewer transplants in Era 3 (P<0.001). The cumulative incidence of explant and of death whilst on VAD support had not changed significantly with time (P = 0.26 and P = 0.40, respectively).

Of the 45 patients with BiVAD support, in 15 cases the RVAD support was explanted. In a further 15 cases, the patient was transplanted, and the remaining 15 patients died on support.
Survival adjusted for risk
Hazard ratios for death for implants in Eras 1, 2, and 3, adjusted for generation of VAD, age, gender, explant without a transplant, and HTx after VAD support are summarized in Table 3. Similar to the unadjusted analysis, there was no significant difference in risk by era (P = 0.58), whilst there were statistically significant differences by device generation (P = 0.0026). The risk of death for recipients of third-generation devices was significantly less than for recipients of first- and second-generation devices (P<0.017 after Bonferroni correction), while, in contrast, the risk of death for recipients of first- and second-generation devices was similar (P = 0.66 after Bonferroni correction). As anticipated, the risk of death increased with age. Compared with a patient on VAD support awaiting HTx, a transplanted VAD patient was at significantly increased risk of death in the month following HTx (hazard ratio 11.1, 95% CI 4.41–27.7). Thereafter, the risk reduced; in the period 31–90 days post-HTx, the risk of death was similar to the risk of death on VAD support, whereas after 90 days post-HTx the risk of death was lower than for patients on VAD support without a transplant (hazard ratio 0.12, 95% CI 0.04–0.39).
| Variable | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
| Era | 0.58 | ||
| 1 | 1.00 | – | |
| 2 | 1.32 | 0.74–2.36 | |
| 3 | 1.43 | 0.70–2.88 | |
| Generation of VAD device | 0.0026 | ||
| First | 1.00 | – | |
| Second | 0.70 | 0.40–1.24 | |
| Third | 0.34 | 0.18–0.65 | |
| Age (per 10 years) | 1.27 | 1.07–1.49 | 0.004 |
| Female gender | 1.24 | 0.75–2.05 | 0.40 |
| Explant without transplant | 0.25 | 0.06–1.08 | 0.063 |
| Transplant | <0.0001 | ||
| 0–7 days post-HTx | 11.1 | 4.41–27.7 | |
| 8–30 days post-HTx | 7.68 | 3.38–17.5 | |
| 31–90 days post-HTx | 1.43 | 0.43–4.74 | |
| 90+ days post-HTx | 0.12 | 0.04–0.39 |
- a CI, confidence interval; HTx, heart transplantation; VAD, ventricular assist device.
Causes of death
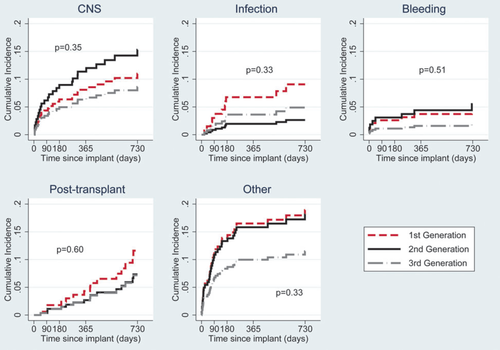
At the time of analysis, 103 of the 247 study patients had died. Causes of death by generation of device are shown in Table 4, and the cumulative incidence of death due to bleeding, infection, central nervous system (CNS) causes, other causes, and after HTx are shown in Figure 5. Deaths due to CNS causes were less common with first-generation devices, but statistically there were no significant differences between the groups.
| Device generation | ||||
|---|---|---|---|---|
| First | Second | Third | Total | |
| Person-years of follow-up | 97.2 | 161.4 | 164.6 | 423.2 |
| Primary cause of death | n (%) | n (%) | n (%) | n (%) |
| CNS | 5 (18.5) | 12 (30.0) | 9 (25.0) | 26 (25.2) |
| Infection | 4 (14.8) | 2 (5.0) | 5 (13.9) | 11 (10.7) |
| Bleeding | 2 (7.4) | 4 (10.0) | 2 (5.6) | 8 (7.8) |
| Cardiovascular | 3 (11.1) | 3 (7.5) | 2 (5.6) | 8 (7.8) |
| Multiorgan failure | 4 (14.8) | 4 (10.0) | 0 | 8 (7.8) |
| Embolic | 1 (3.7) | 2 (5.0) | 1 (2.8) | 4 (3.9) |
| Device malfunction | 0 | 1 (2.5) | 1 (2.8) | 2 (1.9) |
| Thrombosis | 0 | 0 | 1 (2.8) | 1 (1.0) |
| Post-HTx | 7 (25.9) | 7 (17.5) | 7 (19.4) | 21 (20.4) |
| Other | 0 | 2 (5.0) | 6 (16.7) | 8 (7.8) |
| Unknown | 1 (3.7) | 3 (7.5) | 2 (5.6) | 6 (5.8) |
| Total | 27 | 40 | 36 | 103 |
- a CNS, central nervous system; HTx, heart transplantation; VAD, ventricular assist device.
Discussion
This report, from a comprehensive UK national database of long-term mechanical circulatory support for advanced HF, has documented changes in clinical practice with increasing activity and a progressive shift from the use of first- to third-generation LVADs. Despite the initial indication being bridge to transplantation, the scarcity of suitable donor hearts has resulted in a lengthening of the duration of support, with the longest surviving patient now being on VAD therapy for >6 years. The probability of receiving a HTx whilst on mechanical circulatory support has declined progressively due to a decrease in the number of suitable donor hearts, and survival has significantly improved in association with the newest generation of device.
The UK VAD registry collects data from all devices and reflects current LVAD technology and clinical use, whereas the INTERMACS registry only includes devices after final FDA approval.7,10,11 European registries are voluntary, whereas the UK database is comprehensive and data collection is linked to NHS reimbursement. The data are independently validated; case note validation is performed at the centres and computerized validation checks are performed during data entry. Thus, this report is unique in providing a comprehensive national picture of clinical practice including newer devices.
Devices implanted during Era 1 were mainly large first-generation pulsatile volume displacement pumps. These were subsequently replaced by smaller second-generation axial impeller devices, and, currently, the majority of VADs implanted are third-generation devices. These are smaller, bearing centrifugal pumps, which can be implanted within the pericardium, thereby obviating the need for further surgery to create a space or pocket to accommodate the device. The drive lines of second- and third-generation devices are smaller and more flexible, which may reduce the risk of drive line infection. Our data show a progressive improvement in survival. There have been few deaths due to infection in patients with third-generation devices despite the median duration of support being longer in this group than in patients implanted with first- and second-generation devices. Similar improvements in survival have been seen in other randomized and non-randomized studies.5,7,12
Survival has improved with time, albeit not significantly, with overall survival in Era 3 approaching 70% at 1 year. This compares with a 1-year survival after HTx of 81.6% over the same period. One factor associated with improved survival is the change in LVAD technology. The REMATCH trial established a survival benefit for patients implanted with a first-generation device when compared with medical therapy for advanced HF.4 The HeartMate II trial showed a further improvement in survival with a second-generation device.5 The ADVANCE study has recently shown similar survival at 6 months after the use of a third-generation device as after second-generation devices in the bridge to transplantation indication.13 In contrast, the present study found better survival with third-generation devices. Possible reasons for this difference include the longer period of observation in this study (survival diverged progressively, Figure 3) and the low rate of transplantation in the UK compared with the USA, minimizing the effect of transplantation on survival though informative censoring. Further studies are necessary to confirm or refute this potentially important observation.
In the present study, actuarial 1-year survival in an unselected group of patients with third-generation devices was 77%, which is similar to survival rates reported in the USA.7 However, survival for the cohort as a whole—69% at 1 year and 56% at 2 years—is lower than that reported in some clinical trials, probably due to the unselected nature of the cohort, the longer follow-up, and the higher proportion of patients in INTERMACS classes 1 and 2.7,14
While survival improved with time, this was associated with device generation rather than era per se. This conclusion should be regarded as preliminary because there may have been other changes in clinical practice which were not captured in the database.
Subsequent HTx or VAD explant (following recovery of myocardial function)15 had a significant impact on patient survival. After the initial increased risk, HTx lowered the hazard for death, reflecting the relatively high risk of late mortality with ongoing VAD support, compared with HTx. Similarly, the risk of death after explant in patients who had experienced a recovery in myocardial function was lower than for patients with ongoing VAD support.
Causes of death during VAD support were most commonly CNS or infective events. The cause varied depending upon time since VAD implantation. Most early deaths were due to bleeding, cerebrovascular events (including intracranial bleeding), and sepsis.
The median duration of support has increased significantly over time; 141 days in Era 1 to 533 days in Era 3. This increase is partly due to improved survival on support and partly because continued support was necessitated by a lack of available donor hearts for transplantation.
Implications for practice
The use of VAD therapy as a bridge to heart transplantation has increased in the UK, and VAD support has become a de facto alternative to transplantation for many of these patients due to the scarcity of suitable donor hearts. Third-generation devices are now preferred to first- and second-generation devices due to their ease of surgical implantation and, potentially, improved medium-term survival. Device durability is excellent, with few deaths due to device malfunction. Although the long-term outcome with a VAD is not as good as after HTx, the results are improving and this treatment is not limited by donor availability.
Limitations
This study is limited by its observational nature. There was no standardization of practice across centres, and treatment was determined by the treating physician and surgeon. However, we have used data from a comprehensive national registry, thereby avoiding bias due to case selection or in the presentation of results. To date, this is the most complete study of VAD outcomes from a single country.
Risk adjustment is always limited; observed differences in survival could be due to confounding with measured and unobserved variables. Each device generation includes a variety of devices with different characteristics which could influence the clinical outcome. However, the limited number of cases precluded comparisons between individual devices.
Conclusion
Donor heart availability has decreased, resulting in fewer transplants and an increase in the number of VADs implanted into patients with advanced HF. VAD technology has evolved rapidly and there has been a progressive improvement in outcome. VAD therapy has become a medium-term alternative to HTx, with survival rates at 1 year for those with third-generation devices approaching those after HTx. However, there still appears to be a long-term survival benefit for VAD patients who subsequently undergo HTx.
Funding
The National Specialized Commissioning Team, NHS UK, fund the UK Cardiothoracic Transplant Audit. This work was carried out independently of the funder. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the National Specialized Commissioning Team, NHS or the UK Department of Health.
Conflict of interest: none declared.
Authors' contributions: A.E. designed the study with C.A.R., J.P. and N.R.B. A.E. carried out some of the statistical analysis under the guidance of C.A.R. and drafted the report with C.A.R. and N.R.B. C.A.R. designed the study with A.E., J.P., and N.R.B., led and carried out some of the statistical analysis and drafted the report with A.E and N.R.B. J.P. designed the study with N.R.B., C.A.R., A.E., and critically reviewed the data and the report. R.T. extracted the data from the national VAD database, and critically reviewed the data and the report. G.M., S.S., A.S., S.T., and N.Y. critically reviewed the data and the report. N.R.B.led the project. He designed the study with A.E., C.A.R., and J.P., critically reviewed the data, and drafted the report with A.E. and C.A.R.
Appendix 1
Cardiothoracic Transplant Audit Steering Group
Dr Nicholas Banner, Harefield Hospital, Harefield UB9 6JH (Chair); Mr Peter Braidley, Northern General Hospital, Sheffield S5 7AU; Dr Mike Burch, Great Ormond Street Hospital for Children, London WC1N 3JH; Mr Stephen Clark, Freeman Hospital, Newcastle upon Tyne NE7 7DN; Professor Dave Collett, NHS Blood and Transplant, Bristol BS34 8RR; Dr David Cromwell, Royal College of Surgeons of England, London WC2A 3PN; Dr Kate Haire, NSCT, NHS Specialised Services, London SW12 6QT; Mr Jorge Mascaro, Queen Elizabeth Hospital, Birmingham B15 2TH; Dr Jayan Parameshwar, Papworth Hospital, Papworth Everard CB3 8RE; Dr Mark Petrie, Golden Jubilee National Hospital, Glasgow G81 4DY; Mr Andre Simon, Harefield Hospital, Harefield UB9 6JH; Mr Steven Tsui, Papworth Hospital, Papworth Everard CB3 8RE; Professor Nizar Yonan, Wythenshawe Hospital, Manchester M23 9LT.
VAD Forum
Dr Nicholas Banner, Harefield Hospital, Harefield UB9 6JH; Ms Katherine Collins, NHS National Services Scotland, Edinburgh EH12 9EB; Dr Kate Haire, NSCT, NHS Specialised Services, London SW12 6QT (Chair); Mr Saleem Haj-Yahia, Harefield Hospital, Harefield UB9 6JH; Dr Guy MacGowan, Freeman Hospital, Newcastle upon Tyne NE7 7DN; Mr Jorge Mascaro, Queen Elizabeth Hospital, Birmingham B15 2TH; Dr Jayan Parameshwar, Papworth Hospital, Papworth Everard CB3 8RE; Dr Mark Petrie, Golden Jubilee National Hospital, Glasgow G81 4DY; Mr Steven Shaw, Wythenshawe Hospital, Manchester M23 9LT; Professor Stephan Schueler, Freeman Hospital, Newcastle upon Tyne NE7 7DN; Mr Andre Simon, Harefield Hospital, Harefield UB9 6JH; Mr Steven Tsui, Papworth Hospital, Papworth Everard CB3 8RE; Mr John Townsend, Queen Elizabeth Hospital, Birmingham B15 2TH; Mr Rajamiyer Venkateswaran, Wythenshawe Hospital, Manchester M23 9LT; Mr Ian Wilson, Queen Elizabeth Hospital, Birmingham B15 2TH; Mr Mike Winter, NHS National Services Scotland, Edinburgh EH12 9EB; Professor Nizar Yonan, Wythenshawe Hospital, Manchester M23 9LT.
Appendix 2
UK centres contributing data to this report
Freeman Hospital, Newcastle; Papworth Hospital; Harefield Hospital, London; University Hospitals Birmingham; Wythenshawe Hospital, Manchester; Golden Jubilee Hospital, Glasgow




