Haemoconcentration, renal function, and post-discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial
Abstract
Aims
Haemoconcentration has been studied as a marker of decongestion in patients with hospitalization for heart failure (HHF). We describe the relationship between haemoconcentration, worsening renal function, post-discharge outcomes, and clinical and laboratory markers of congestion in a large multinational cohort of patients with HHF.
Methods and results
In 1684 patients with HHF with ejection fraction (EF) ≤40% assigned to the placebo arm of the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial, absolute in-hospital haematocrit change was calculated as the change between baseline and discharge or day 7 (whichever occurred first). Patient characteristics, changes in renal function, and outcomes over a median follow-up of 9.9 months were compared by in-hospital haematocrit change. Overall, 26% of patients had evidence of haemoconcentration (i.e. ≥3% absolute increase in haematocrit). Patients with greater increases in haematocrit tended to have better baseline renal function. Haemoconcentration correlated with greater risk of in-hospital worsening renal function, but renal parameters generally returned to baseline within 4 weeks post-discharge. Patients with haemoconcentration were less likely to have clinical congestion at discharge, and experienced greater in-hospital decreases in body weight and natriuretic peptide levels. After adjustment for baseline clinical risk factors, every 5% increase of in-hospital haematocrit change was associated with a decreased risk of all-cause death [hazard ratio (HR) 0.81, 95% confidence interval (CI) 0.70–0.95]. Haematocrit change was also associated with decreased cardiovascular mortality or heart failure (HF) hospitalization at ≤100 days post-randomization (HR 0.73, 95% CI 0.71–0.76).
Conclusion
In this large cohort of patients with HHF with reduced EF, haemoconcentration was associated with greater improvements in congestion and decreased mortality and HF re-hospitalization despite an increased risk of in-hospital worsening renal function.
Introduction
Hospitalization for heart failure (HF) occurs >1 million times per year in the USA alone, and represents one of the strongest predictors of poor prognosis.1,2 The most common reason for hospitalization in these patients is worsening chronic HF with pulmonary or systemic congestion.3–5 While standard in-hospital therapies, namely diuretics, significantly improve symptoms in the majority of patients, many HF patients are discharged with persistent congestion.4,5
Given the prognostic significance associated with the degree of congestion at discharge,6 novel objective endpoints for inpatient decongestion are needed to guide therapy and discharge decision-making. In this regard, previous studies have explored the role of haemoconcentration and intravascular volume contraction in patients with hospitalization for HF (HHF).7–9 Moreover, while previous studies have demonstrated an association between worsening renal function (WRF) and increased mortality among patients with HHF,10,11 several recent reports have challenged this notion.12–14 The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial database affords the opportunity to perform a detailed characterization of HHF patients with reduced ejection fraction (EF) by degree of in-hospital haemoconcentration and to describe the relationship between haemoconcentration, worsening renal function, post-discharge outcomes, and clinical and laboratory markers of congestion in a large multinational cohort of HHF patients.
Methods
Study Design
The study design15 and primary results of the EVEREST programme have been previously reported.16,17 Briefly, the EVEREST trial was a prospective, multicentre, multinational, randomized, double-blind, placebo-controlled clinical trial investigating the role of oral tolvaptan on short- and long-term clinical outcomes among patients with HHF. Notably, patients were enrolled within 48 h of admission at 359 clinical sites from North America, South America, and Europe from 2003 to 2006. Patients over the age of 18 who were hospitalized for worsening HF with EF ≤40% and New York Heart Association (NYHA) class III or IV functional status presenting with ≥2 signs/symptoms of fluid overload (i.e. dyspnoea, pitting oedema, and jugular venous distension) were eligible for enrolment. Exclusion criteria relevant for the present study included haemoglobin level <9 g/dL, serum creatinine (SCr) >3.5 mg/dL, or co-morbid conditions with expected survival <6 months.
Institutional review boards or ethics committees at each centre approved the study protocol. In addition, patients provided written informed consent for participation. Background HF therapy was left to the discretion of the treating physician, but guideline-based recommendations for optimal medical management were included in the study protocol.
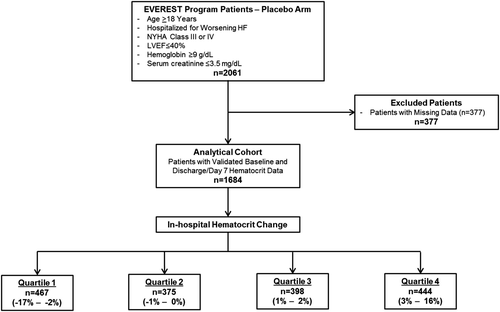
Since oral tolvaptan has been shown to decrease body weight and increase renal excretion of free water, the present post-hoc analysis included patients assigned to the placebo arm only. Complete blood counts including haematocrit, basic chemistries, body weight, vital signs, and physical examination data were collected at randomization and hospital day 7 or discharge (whichever occurred first). Patients with missing baseline or discharge/day 7 haematocrit data were excluded. Other potential markers of haemoconcentration, such as albumin and total protein level, were not examined in the present study. Figure 1 provides an overview of the patient selection for this post-hoc analysis.
Definitions
All-cause mortality (ACM) and the composite of cardiovascular mortality or hospitalization for HF (CVM/HHF), the two EVEREST co-primary endpoints, were also selected for the present study. Endpoints were analysed as time to first event. An independent, blinded clinical events committee adjudicated specific causes of death, hospitalization, and unscheduled outpatient visits. Median follow-up was 9.9 months.
In-hospital haematocrit change was defined as haematocrit at baseline subtracted from haematocrit at discharge/day 7 (whichever occurred first). Consistent with a previous EVEREST analysis,11 worsening SCr was defined as an absolute increase in SCr ≥0.3 mg/dL;18–20 worsening blood urea nitrogen (BUN) was defined as a ≥25% increase;21 and worsening estimated glomerular filtration rate (eGFR) was defined as a ≥25% decrease.14,21 For all measurements of change in renal function, body weight, and natriuretic peptide levels, data reflect change from randomization to discharge/day 7 (whichever occurred first).
Statistical analysis
For descriptive purposes, patients were grouped into quartiles of in-hospital haematocrit change. Baseline demographics, medical history, medication history, physical exam, and laboratory findings were compared across quartiles using χ2, analysis of variance (ANOVA), and Kruskal–Wallis tests where appropriate. All continuous variables were reported as mean ± standard deviation if normally distributed or median [interquartile range (IQR)] if non-normally distributed. The frequencies of in-hospital worsening SCr, BUN, and eGFR were calculated across quartiles.
The primary predictor of the present study was in-hospital haematocrit change, expressed as a continuous variable. Univariate and multivariable Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the primary predictor (as a continuous variable). The linearity and proportional hazards assumptions were tested for both primary endpoints by Kolmogorov-type supremum tests. For the composite endpoint, the proportional hazards assumption did not hold (P < 0.004), so the follow-up period was divided into two phases at 100 days post-randomization (cut-off point established by visual inspection of Standardized Score Process plots). Kaplan–Meier curves were constructed for in-hospital haematocrit change quartiles, and time to first event was compared using log-rank tests.
Multivariable models were adjusted for 26 pre-selected baseline covariates known to influence clinical endpoints and utilized in previous EVEREST analyses: age, sex, geographic region, ischaemic HF aetiology, NYHA functional class IV, EF, B-type natriuretic peptide (BNP), N-terminal-proBNP (NT-proBNP), serum sodium, BUN, SCr, eGFR, systolic blood pressure, QRS duration on enrolment ECG, haematocrit, baseline co-morbidities (self-reported history of diabetes mellitus, renal insufficiency, hypertension, coronary artery disease, chronic obstructive pulmonary disease, atrial fibrillation on baseline ECG, and history of HF hospitalization), and baseline medication use [angiotensin-converting enzyme (ACE) inhibitors/ angiotensin II receptor blockers (ARBs), beta-blockers, mineralocorticoid receptor antagonists (MRAs), and digoxin]. Multicollinearity between the primary predictor and control variables did not significantly threaten the results of the multivariable models (tolerance = 0.60). The multiple imputation procedure (fully conditional specification methods as implemented in MI and MIANALYZE procedures in SAS (SAS Institute, Cary, NC, USA) was used for missing covariate data (<5% for all variables). Pre-specified interaction analysis was performed between the primary predictor and baseline haematocrit quartile. All statistical analyses were performed using SAS version 9.3 (SAS Institute), and two-tailed P < 0.05 was considered to be statistically significant.
Results
Baseline characteristics
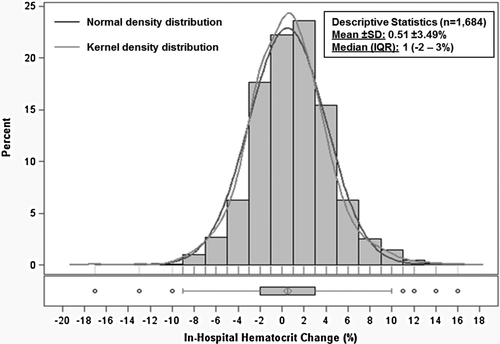
The final analytical cohort included 1684 patients after excluding those with missing data. No patients in this cohort received a blood transfusion during hospitalization. The distribution of in-hospital haematocrit change was approximately normal with mean +0.51 ± 3.49% and median +1% (IQR –2% to 3%) (Figure 2). Table 1 describes the baseline characteristics for patients by quartile of in-hospital haematocrit change. Together, quartiles 2 and 3 comprised slightly less than half the study population and included patients with relatively stable haematocrit during hospitalization (–1% to +2%). Patients in quartile 1 were considered ‘haemodilutors’ (in-hospital haematocrit change –2% or less) while those in quartile 4 (i.e. 26% of patients) were considered ‘haemoconcentrators’ (in-hospital haematocrit change ≥3%). Patients with greater in-hospital increase in haematocrit tended to have lower baseline haematocrit and were more likely to be taking an MRA and have dyspnoea at baseline. They were also more likely to have better baseline renal function characterized by lower baseline SCr and BUN and higher eGFR.
| Characteristics | Quartile 1 (n = 467) | Quartile 2 (n = 375) | Quartile 3 (n = 398) | Quartile 4 (n = 444) | P-value |
|---|---|---|---|---|---|
| In-hospital haematocrit change range (%) | –17% to –2% | –1% to 0% | 1% to 2% | 3% to 16% | – |
| Baseline haematocrit (%) | 44 ± 6 | 42 ± 5 | 42 ± 6 | 41 ± 5 | <0.001 |
| Demographics | |||||
| Age (years) | 66 ±12 | 66 ±12 | 64 ±12 | 65 ±12 | 0.068 |
| Male | 349 (75) | 287 (77) | 306 (77) | 338 (76) | 0.886 |
| Ischaemic heart failure aetiology | 316 (69) | 240 (65) | 263 (67) | 287 (66) | 0.644 |
| NYHA class IV | 196 (42) | 133 (36) | 150 (38) | 190 (43) | 0.095 |
| Baseline laboratory values and clinical characteristics | |||||
| Systolic blood pressure (mmHg) | 121 ± 19 | 120 ± 19 | 120 ± 19 | 122 ± 20 | 0.299 |
| Diastolic blood pressure (mmHg) | 73 ± 12 | 72 ± 13 | 73 ± 12 | 74 ± 13 | 0.090 |
| Heart rate (b.p.m.) | 80 ± 17 | 77 ± 14 | 81 ± 15 | 81 ± 16 | 0.001 |
| Body weight (kg) | 83 ± 19 | 83 ± 17 | 85 ± 19 | 84 ± 18 | 0.079 |
| Dyspnoea | 421 (91) | 326 (88) | 353 (91) | 415 (95) | 0.014 |
| Rales | 391 (85) | 310 (84) | 312 (81) | 349 (80) | 0.161 |
| Jugular venous distention ≥10 cm | 108 (24) | 100 (27) | 98 (25) | 131 (30) | 0.163 |
| Peripheral oedemaa | 378 (82) | 307 (83) | 313 (81) | 353 (80) | 0.845 |
| Ejection fraction (%) | 28 ± 8 | 28 ± 8 | 27 ± 8 | 28 ± 8 | 0.081 |
| BNP (pg/mL)b | 732 (284–1484) | 713 (322–1609) | 623 (260–1505) | 766 (357–1411) | 0.584 |
| NT-proBNP (pg/mL)c | 5449 (1812–11642) | 4327 (1883–8191) | 4246 (2271–8322) | 4459 (2595–7850) | 0.483 |
| Serum creatinine (mg/dL) | 1.3 (1.1–1.7) | 1.3 (1.0–1.5) | 1.2 (1.0–1.5) | 1.2(1.0–1.5) | 0.001 |
| Glomerular filtration rate (mL/min/1.73 m2) | 53 (38–67) | 55 (42–69) | 56 (44–72) | 58 (43–72) | <0.001 |
| Serum urea nitrogen (mg/dL) | 28 (20–40) | 26 (19–34) | 25 (20–31) | 24 (18–33) | <0.001 |
| Serum sodium (mEq/L) | 140 (137–142) | 140 (137–143) | 140 (138–143) | 140 (137–142) | 0.490 |
| QRS duration of baseline ECG (ms) | 122 (97–150) | 124 (97–154) | 123 (98–153) | 122 (98–146) | 0.434 |
| Atrial fibrillation/flutter on baseline ECG | 135 (29) | 97 (26) | 125 (31) | 135 (30) | 0.363 |
| Past medical history | |||||
| Hypertension | 344 (74) | 257 (69) | 274 (69) | 311 (70) | 0.321 |
| Hyperlipidaemia | 216 (47) | 174 (47) | 185 (47) | 185 (42) | 0.453 |
| CAD | 335 (72) | 264 (70) | 285 (72) | 305 (69) | 0.747 |
| Previous HF hospitalization | 361 (78) | 296 (79) | 316 (79) | 349 (79) | 0.937 |
| Diabetes mellitus | 165 (35) | 155 (41) | 137 (34) | 159 (36) | 0.181 |
| Chronic kidney disease | 135 (29) | 90 (24) | 88 (22) | 113 (26) | 0.127 |
| COPD | 52 (11) | 38 (10) | 34 (9) | 33 (7) | 0.234 |
| Baseline medication use | |||||
| Diuretics | 451 (97) | 367 (98) | 389 (98) | 428 (97) | 0.567 |
| Beta-blockers | 324 (70) | 261 (70) | 281 (71) | 315 (71) | 0.927 |
| ACE inhibitor/ARB | 394 (85) | 307 (82) | 347 (87) | 385 (87) | 0.099 |
| Mineralocorticoid receptor antagonist | 239 (51) | 201 (54) | 242 (61) | 270 (61) | 0.004 |
| Digoxin | 221 (47) | 174 (46) | 217 (55) | 224 (51) | 0.083 |
| Clinical endpoints | |||||
| All-cause mortality | 139 (30) | 97 (26) | 86 (22) | 89 (20) | 0.003 |
| CV mortality or HF hospitalization | 201 (43) | 144 (38) | 144 (36) | 158 (36) | 0.087 |
| CV mortality | 109 (23) | 73 (20) | 65 (16) | 68 (15) | 0.009 |
| HF hospitalization | 135 (29) | 102 (27) | 103 (26) | 111 (25) | 0.574 |
- a Data are expressed as mean ± SD, median (interquartile range), or n (%).
- b ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BNP, B-type natriuretic peptide; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; HF, heart failure; NT-proBNP, N-terminal-proBNP; NYHA, New York Heart Association.
- c aSlight, moderate, or marked pedal or sacral oedema.
- d bData available for 347, 283, 291, and 327 patients in quartiles 1–4, respectively.
- e cData available for 150, 135, 143, and 143 patients in quartiles 1–4, respectively.
Changes in renal function and congestion
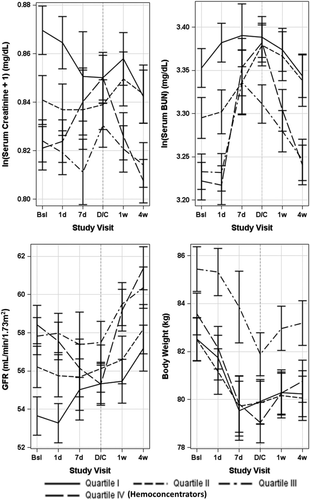
Haemoconcentrators were more likely to experience WRF, including worsening SCr, BUN, and eGFR (Table 2). The risk of WRF was relatively stable across quartiles 1–3. When parameters of renal function were examined longitudinally, within 4 weeks post-discharge, haemoconcentrators tended to have improvement of renal function approximately back to baseline levels (Figure 3).
| Event | Quartile 1 (n = 467) | Quartile 2 (n = 375) | Quartile 3 (n = 398) | Quartile 4 (n = 444) | P-value |
|---|---|---|---|---|---|
| Renal function | |||||
| Worsening serum creatininea | 59 (13) | 39 (11) | 45 (12) | 89 (20) | <0.001 |
| Worsening blood urea nitrogenb | 139 (31) | 111 (30) | 113 (29) | 181 (41) | <0.001 |
| Worsening glomerular filtration ratec | 50 (11) | 36 (10) | 31 (8) | 79 (18) | <0.001 |
| Measures of congestion at discharge/day 7 | |||||
| Mean change in body weightd | –2.6% | –2.8% | –3.4% | –4.8% | <0.001 |
| Mean change in Ln(BNP)d | –0.28 | –0.25 | –0.33 | –0.62 | <0.001 |
| Mean change in Ln(NT-proBNP)d | –0.33 | –0.43 | –0.40 | –0.53 | <0.001 |
| BNP (pg/mL), median (IQR)e | 521 (208–1127) | 509 (219–1176) | 488 (196–1194) | 382 (167–787) | 0.003 |
| NT-proBNP (pg/mL), median (IQR)f | 3146 (1120–7800) | 3027 (1232–5933) | 2894 (1558–5675) | 2466 (1175–4511) | 0.352 |
| Dyspnoea | 117 (25) | 95 (25) | 102 (26) | 71 (16) | 0.003 |
| Rales | 122 (26) | 91 (24) | 87 (22) | 70 (16) | 0.001 |
| Jugular venous distention ≥10 cm | 27 (6) | 27(7) | 23 (6) | 15 (3) | 0.107 |
| Peripheral oedemag | 132 (28) | 107 (29) | 97 (24) | 76 (17) | <0.001 |
- a Data are expressed as n (%) unless otherwise specified.
- b IQR, interquartile range.
- c aDefined as ≥0.3 mg/dL increase at discharge/day 7 compared with baseline.
- d bDefined as ≥25% increase at discharge/day 7 compared with baseline.
- e cDefined as ≥25% decrease at discharge/day 7 compared with baseline.
- f dDefined as discharge/day 7 value compared with baseline value. For change in body weight, measurements from in-hospital days 6, 5, 4, and 3 were also used when discharge/day 7 data were not available (10 patients). For mean change in Ln(BNP), data were available for 325, 262, 269, and 309 patients in quartiles 1–4, respectively. For mean change in Ln(NT-proBNP), data were available for 107, 83, 95, and 104 patients in quartiles 1–4, respectively.
- g eData available for 355, 281, 297, and 340 patients in quartiles 1–4, respectively.
- h fData available for 148, 132, 140, and 142 patients in quartiles 1–4, respectively,
- i gSlight, moderate, or marked pedal or sacral oedema.
Haemoconcentrators experienced greater decongestion, as evidenced by greater in-hospital decreases in body weight and natriuretic peptide levels. They were also less likely to have dyspnoea, rales, and peripheral oedema at the time of discharge/day 7. Discharge/day 7 levels of BNP, but not NT-proBNP, were significantly lower in haemoconcentrators. Regardless of haematocrit change quartile, all patients tended to gain weight within 4 weeks post-discharge.
Post-discharge outcomes
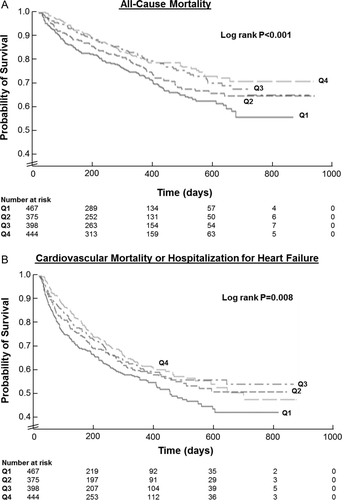
A total of 411 ACM and 647 CVM/HHF events occurred during a median follow-up of 9.9 months. Outcome analysis by 5% absolute increases of in-hospital haematocrit change is displayed in Table 3. Univariate analysis found that increases in haematocrit were associated with decreased risk of ACM (HR 0.79, 95% CI 0.68–0.90). Haematocrit change was also associated with the composite endpoint at ≤100 days post-randomization (HR 0.74, 95% CI 0.63–0.86), but not >100 days (HR 1.02, 95% CI 0.87–1.19). Times to first event between patients stratified by haematocrit change quartile were significantly different by the Kaplan–Meier method for ACM (Figure 4A, log rank P < 0.001) and CVM/HHF (Figure 4B, log rank P = 0.008).
| Outcome | Unadjusted HR (95% CI) | Adjusted HRb (95% CI) | |
|---|---|---|---|
| ACM | 0.79 (0.68–0.90) | 0.81 (0.70–0.95) | |
| CVM/HHFc | ≤100 days | 0.74 (0.63–0.86) | 0.73 (0.71–0.76) |
| >100 days | 1.02 (0.87–1.19) | 0.99 (0.96–1.02) |
- a ACM, all-cause mortality; CVM, cadiovascular mortality; HHF, hospitalization for heart failure.
- b aHazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for every 5% increase of baseline-to-discharge/day 7 absolute haematocrit change.
- c bAdjusted for age (years), sex, geographic region, ischaemic heart failure aetiology, NYHA functional class IV, EF (%), BNP (pg/mL), NT-proBNP (pg/mL), serum sodium (mEq/L), blood urea nitrogen (mg/dL), serum creatinine (mg/dL), estimated glomerular filtration rate (mL/min), systolic blood pressure (mmHg), QRS duration on baseline ECG (ms), haematocrit (%), self-reported history of diabetes mellitus, renal insufficiency, hypertension, CAD, COPD, AF on baseline ECG, history of heart failure hospitalization, and baseline use of ACE inhibitor/ARB, beta-blockers, mineralocorticoid receptor antagonist, and digoxin.
- d cFor the composite endpoint, the proportional hazards assumption did not hold (P < 0.004); therefore, the follow-up period was divided into two phases at 100 days post-randomization (cut-off point established by visual inspection of Standardized Score Process plots).
After adjustment for baseline clinical risk factors, haematocrit change remained an independent predictor of ACM (HR 0.81, 95% CI 0.70–0.95). Again, haematocrit change was associated with the composite endpoint ≤100 days post-randomization (HR 0.73, 95% CI 0.71–0.76), but not >100 days (HR 0.99, 95% CI 0.96–1.02). There was no difference in the effect of continuous haematocrit change by baseline haematocrit quartile for ACM (P = 0.78) or CVM/HHF (≤100 days P = 0.98; >100 days P = 0.77).
Discussion
In a large contemporary cohort of patients with HHF receiving guideline-directed medical therapies, approximately a quarter of patients experienced substantial in-hospital increases in haematocrit (i.e. ≥3% absolute increase in haematocrit). Overall, patients in higher quartiles of in-hospital haematocrit change were more likely to have better baseline renal function. Haemoconcentrators were more likely to experience in-hospital WRF, but renal function tended to return to baseline within 4 weeks post-discharge. Furthermore, haemoconcentration correlated with greater in-hospital decreases in body weight and natriuretic peptide levels and less congestion at time of discharge. Lastly, greater in-hospital increases in haematocrit were independently predictive of decreased ACM across the duration of follow-up and decreased CVM/HHF in the early post-discharge period.
Our findings reaffirm and add to the recently published results from the Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) trial in multiple ways.9 While those authors also found haemoncentration, defined as absolute in-hospital increases in haemoglobin, to correlate with favourable prognosis despite a decrease in renal function, our study includes data regarding in-hospital changes in natriuretic peptide levels, defines the association between haematocrit change and several commonly used markers of congestion at baseline and discharge, and describes the temporal change in multiple measures of renal function longitudinally across in-hospital and post-discharge periods. Furthermore, characterization of post-discharge outcome in the PROTECT study was limited to 180 day ACM, while EVEREST offered the ability to describe the relationship between haemoconcencentration and the composite of CVM/HHF over a median follow-up of 9.9 months. Additionally, our data are consistent with those of Testani et al. in 336 patients from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial where patients with haemoconcentration experienced greater net weight loss and substantially lower risk of mortality despite increased risk for WRF.7 Similar to our findings and those from PROTECT, patients with haemoconcentration tended to have better baseline renal function. However, it is notable that the definition of haemoconcentration used by Testani differed from that in the present study and included albumin and total protein values, in addition to haematocrit data. Interestingly, when haematocrit change was analysed separately, it failed to predict post-discharge outcomes or WRF, while albumin and total protein continued to be predictive when analysed in isolation. Our findings are also different from those of a small single-centre study performed by Davila et al.8 which found in-hospital increases in haemoglobin associated with WRF, but not mortality.
The present study found significant differences in baseline haematocrit across continuous haematocrit change quartiles. An inverse relationship between severity of anaemia and clinical outcomes in chronic HF has been well described22 and there are conflicting data in HHF.9,23 Notably, one investigation in ambulatory patients found anaemia secondary to haemodilution to be associated with worse overall prognosis compared with anaemia from decreased red blood cell mass, further suggesting the negative influence of congestion.24 In the current analysis, while haemoconcentrators tended to have the lowest haematocrit levels at time of enrolment, these patients experienced a significant reduction in post-discharge events with improvement in the dilutional component of anaemia. This effect was seen regardless of baseline haematocrit quartile.
Cardiorenal interactions in HHF have become increasingly recognized, and achieving adequate control of congestion with simultaneous preservation of renal function has been a goal of inpatient management. Many studies have correlated renal parameters at time of admission and adverse outcomes in HHF.25–27 However, data regarding in-hospital WRF in these patients are less clear, complicated by inconsistent consideration of decongestive therapy.28–31 Studies centred on inpatient decongestion have demonstrated that aggressive fluid removal may be possible without adversely affecting outcomes, irrespective of WRF.7 The Diuretic Optimization Strategies Evaluation (DOSE) trial found higher diuretic dosing associated with greater weight loss and higher incidence of WRF without a difference in 60 day outcome.12 Furthermore, although the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial found greater increases in serum creatinine level with ultrafiltration, this did not result in increased mortality or re-hospitalization.13
Taken together, these data suggest that change in congestion status is perhaps a key variable underlying the impact of WRF in this population. The current study found risk of WRF among haemoconcentrators to track with greater improvements in congestion, as evidenced by greater decreases in body weight and natriuretic peptide levels, and overall less clinical congestion at discharge. In addition, the WRF experienced by haemoconcentrators seemed to be a transient phenomenon, and renal parameters returned to baseline within 4 weeks of discharge, suggesting absence of permanent renal injury. Among haemoconcentrators, the change in in-hospital renal function compared with post-discharge may reflect differing intensity of decongestive therapy in the inpatient vs. outpatient settings, with post-hospitalization fluid re-accumulation and/or re-distribution possibly underlying the apparent improvement in renal function after discharge. Moreover, worsening outpatient fluid retention and re-distribution to the intravascular space may partially explain the time-sensitive effect of haemoconcentration on the composite endpoint, with the hypothesis that improved congestion at discharge protects against early cardiac death and re-hospitalization, but that this effect dissipates as intravascular fluid progressively re-accumulates and patients gain weight.
Clinical implications
While approaches have been suggested,32 no systematic method to assess congestion prior to discharge has been proposed by available guidelines.33,34 As a consequence, clinicians may rely on an assortment of grading methods including daily weights, bedside assessment, natriuretic peptide levels, and/or dynamic manoeuvres.32 These measurements each have their strengths and weaknesses and often present conflicting information. Even central venous pressure, a common surrogate for venous congestion, was recently found to track poorly with fluid removal.35,36
Haematocrit represents a widely available, simple, and inexpensive laboratory test that is ordered for virtually every patient with HHF. The findings of the current study favour an intensive inpatient decongestive approach, irrespective of modest worsening in routine renal parameters, in the setting of rising haematocrit. While clinician attention to daily creatinine values is unlikely to abate in the near future, our data support a role for routine consideration of haematocrit changes. Given the current challenges surrounding congestion evaluation and treatment in patients with HHF, new evidence-based endpoints for inpatient fluid removal are needed to guide inpatient therapy and discharge decision-making. In-hospital increase in haematocrit may be a physiologically relevant endpoint that offers objective data on adequacy of decongestion.
Study limitations
A primary limitation of the present analysis is the post-hoc nature of the study design that makes establishing a causal relationship between haemoconcentration and measured outcomes difficult. Although this study was intended to help aid clinical decision-making and better characterize inpatient diuresis endpoints, no specific haematocrit change goals were used. Thus, future clinical trials are required to explore various haemoconcentration goals in comparison with other metrics of decongestion to better define the optimal duration and intensity of inpatient diuresis. Lastly, analyses of other potential and previously studied measurements of haemoconcentration, such as serum total protein and albumin, were not performed. While the authors acknowledge that similar results may have been seen with these markers, the decision to pursue analysis with haematocrit was based on the extent of available EVEREST haematocrit data compared with other laboratory parameters and the wide availability and routine nature of haematocrit testing across clinical practice.
Conclusions
Among patients with HHF with reduced EF, increases in haematocrit were associated with superior in-hospital decongestion and post-discharge mortality and re-hospitalization, despite an increased risk of WRF. These data support the hypothesis that, in the setting of haemoconcentration, possible benefits from improved congestion outweigh any potential detriment from WRF. Haemoconcentration may represent an objective evidence-based endpoint for decongestion, and further studies are encouraged to investigate aggressive decongestive strategies that incorporate haemoconcentration with other markers of congestion.
Funding
Financial and material support for the EVEREST trial was provided by Otsuka, Inc., Rockville, MD. Database management was performed by the sponsor. H.S. conducted all final analyses for this report with funding from the Center for Cardiovascular Innovation, Northwestern University Feinberg School of Medicine, Chicago, IL.
Conflict of interest: M.G. has been a consultant for Abbott Laboratories, Astellas, AstraZeneca, Bayer HealthCare AG, CorThera, Cytokinetics, DebioPharm S.A., Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Johnson & Johnson, Medtronic, Merck, Novartis Pharma AG, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, Takeda Pharmaceutical and Trevena Therapeutics. M.A.K. has been a consultant for Amgen, Johnson & Johnson, Novartis Pharma AG, Otsuka Pharmaceuticals and Merck. All other authors have no conflicts of interest to declare.




