Decrease in dendritic cells in endomyocardial biopsies of human dilated cardiomyopathy
Abstract
Aims
Dendritic cells (DCs) are sentinels of the immune system—their role in myocardial disease is unknown as yet. We investigated their myocardial presence in human dilated cardiomyopathy (DCM).
Methods and results
Endomyocardial biopsies from 72 patients with DCM (EF ∼30%), as well as myocardial specimens from 18 suicide or accident victims were immunohistochemically analysed for myeloid and plasmacytoid DCs, antigen-presenting cells (APCs), and other leucocytes; also tissue fibrosis and apoptosis were histologically quantified. The myocardial viral genome was identified through polymerase chain reaction, and patients underwent clinical follow-up in 3–6 months. We found myocardial DCs of all examined subtypes and maturation stages (fascin, CD11c, CD209, CD83, and CD304), as well as markers for APCs (HLA-DR and CD40) and T-cell activation (CD69) to be significantly decreased in DCM compared with controls. In contrast, regulatory T cells (the GITR epitope), apoptosis (by TUNEL reaction and immunostaining with BCL-2), and a DC chemokine receptor (CCR7) were overexpressed, while no significant differences were observed for macrophages (CD68). Immature myeloid and plasmacytoid DCs strongly correlated with endothelial progenitor cells (CD34), which were similarly reduced in DCM, and inversely correlated with fibrosis. Myeloid DCs were especially reduced in virus-positive biopsies, and their numbers correlated with positive change in EF (ΔEF) at follow-up.
Conclusion
Myocardial DCs are reduced in heart biopsies of symptomatic DCM patients. Such a reduction correlates with an unfavourable short-term outcome in terms of EF, and could result from myocardial tissue damage, cellular death, and insufficient vascularization in chronic heart failure.
Introduction
Dilated cardiomyopathy (DCM) is a primary heart muscle disease of mixed (partly non-genetic) origin, characterized by LV enlargement and systolic dysfunction.1 It is generally assumed that DCM is frequently preceded by acute myocarditis, which is mainly caused by infection of the myocardium with cardiotropic viruses.2 In several studies investigating human biopsy samples, cardiotropic viruses were often found to be present in the myocardium of patients with myocarditis and DCM, although the prognostic impact of such a viral presence and persistence remains controversial.3−5
The use of the endomyocardial biopsy (EMB) for the diagnosis of DCM in individuals with heart failure (HF) is recommended by the European Society of Cardiology (ESC) in its present guidelines for the diagnosis and treatment of HF, when certain clinical scenarios are present.6 The presence of inflammatory cells, tissue necrosis, and fibrosis in EMB indicates myocarditis. Although histological criteria, such as the Dallas criteria, have been proposed to determine an acute, ongoing, or borderline myocarditis, their reported lack of prognostic relevance has raised doubts about their diagnostic significance.7−9
Dendritic cells (DCs) are professional antigen-presenting cells (APCs), which are found in all organ systems, including the myocardium. Several subtypes have been described so far, with the so-called myeloid (m) and plasmacytoid (p) DCs being predominant.10 Following antigen contact, DCs undergo maturation in lymph nodes and subsequently migrate in target organs, where they orchestrate the cellular immune response via activation of T lymphocytes. Beyond T-cell activation, they are also a major source of pro-inflammatory and antiviral cytokines and chemokines, which are necessary for directing the manner of the T-helper cell response.11 Although they are key players in adaptive immunity against antigens, DCs are also known for their crucial role in maintaining peripheral immune tolerance via interaction with regulatory T cells (Tregs).12
In several recent publications in this journal, considerable attention has been given to inflammatory processes and their cellular counterparts in myocarditis and DCM.13–16 Amongst all inflammatory cells, DCs are believed to be key mediators of inflammation during the course of myocarditis, as their maturation can be triggered by cardiotropic viruses.17 Furthermore, in a so-called model of experimental autoimmune myocarditis (EAM), activated bone marrow-derived DCs loaded with α-myosin heavy chain peptide induce acute CD4+ T cell-mediated myocarditis in mice.18 Their role in human myocarditis and its deterioration to DCM nevertheless remain unknown. It is unclear as yet whether DCs and their cellular and humoral mediators are responsible for the induction or suppression of myocardial inflammation during viral myocarditis, thus consecutively inducing or preventing the development of DCM. Recent research on this topic using murine EAM models had come to different conclusions. One group of authors suggested that DCs are responsible for the induction of tissue fibrosis and HF progression.19 On the other hand, another group found that EAM could be prevented by treating mice with DCs transfected with herpes virus entry mediator (HVEM).20 In another recently published study, ablation of DCs in a mice model of acute myocardial ischaemia (ligation of the left anterior descending coronary) led to a deterioration of LV function and remodelling as well as enhanced monocyte/macrophage infiltration and inflammatory cytokines.21 However, data about the frequency and activation of DCs in human myocardium are missing as yet.
In the present study, we used immunostaining techniques to identify and quantify different subtypes of DCs and their other cellular counterparts in myocardial tissue of individuals with clinically suspected and biopsy-confirmed DCM. The results were compared with control myocardial specimens taken from accident or suicide victims without clinically apparent heart disease. Subsequently, we investigated whether there was a correlation between the abundance of myocardial DCs or other immune cells and the severity of DCM in terms of echocardiographic features or tissue fibrosis. This study is the first one to investigate the prognostic impact of the myocardial presence of DCs in humans with DCM.
Methods
Patients
From patients that underwent an EMB and were diagnosed with DCM between January 2007 and December 2011 in our clinic, we found in 72 cases enough preserved biopsy tissue for immunohistochemical analyses. In all patients, CAD (defined as ≥50% stenosis in at least one coronary artery) or other reasons for cardiac dysfunction (valvular heart disease, hypertension, other forms of cardiomyopathy, or systemic disease with cardiac involvement) were excluded by coronary angiography, right heart catheterization, and echocardiography. Standard 2D and M-mode echocardiography were performed 1 day before or on the day of EMB in all patients. For each echocardiogram, LV end-diastolic dimension (LVEDD) and EF were measured by M-mode in the parasternal long-axis view using the leading-edge method. Patients were followed at 3- to 6-month intervals in our outpatient clinic, thereby documenting subsequent echocardiographic parameters. The demographic and clinical characteristics of the patients are shown in Table 1.
| Characteristic | DCM | Controls | P-value |
|---|---|---|---|
| n | 72 | 18 | |
| Age (years) | 46 ± 11 | 41.2 ± 16.8 | 0.313 |
| Male | 57 (79) | 10 (55.6) | 0.042 |
| BMI | 27.4 ± 4.4 | 25.0 ± 3.8 | 0.041 |
| Preceding history of infection | 22 (31) | ||
| Symptom interval to EMB (months) | 3.7 ± 5.4 | ||
| LBBB | 35 (49) | ||
| Medication (%) | |||
| Beta-blockers | 70 (97) | ||
| ACE inhibitors/ARBs | 71 (99) | ||
| Diuretics | 56 (78) | ||
| Aldosterone antagonists | 56 (78) | ||
| Glycosides | 6 (8) | ||
| Clinical presentation | |||
| Angina pectoris | 20 (28) | ||
| Dyspnoea on exertion, NYHA class | |||
| I | 9 (13) | ||
| II | 15 (21) | ||
| III | 37 (51) | ||
| IV | 11 (15) | ||
| Peripheral oedema | 16 (22) | ||
| Syncope | 4 (6) | ||
| Palpitations | 14 (19) | ||
| Atrial fibrillation | 18 (25) | ||
| Echocardiography | |||
| EF, % | 30.2 ± 10.8 | ||
| LVEDD, mm | 62.4 ± 7.9 | ||
| FU interval, months | 4.7 ± 3.1 | ||
| EF-FU, % | 40.9 ± 14.6 | ||
| LVEDD-FU, mm | 60.5 ± 9.1 | ||
| Change EF, % | 8.8 ± 14.9 | ||
| Change LVEDD, mm | –1.2 ± 7.6 |
- a Data are presented as mean values ± standard deviation or number (%) of subjects.
- b BMI, body-mass index; EMB, endomyocardial biopsy; FU, follow-up; LVEDD, left ventricular end-diastolic diameter.
Endomyocardial biopsies
Endomyocardial biopsies were performed when at least one of the specific clinical scenarios stated in the ESC guidelines were present.6 No additional biopsy samples were collected exclusively for the purpose of this study. All patients gave written informed consent. The study was approved by local ethics committee and conducted in compliance with the Declaration of Helsinki.
Endomyocardial biopsies were obtained from the left side of the ventricular septum with a Cordis long sheath bioptome (Cordis Corporation, Miami, FL, USA) via the femoral artery approach. Left and right heart catheterization was performed in the same session. The EMB samples were embedded in TissueTek medium and immediately snap-frozen in liquid nitrogen, then stored at –80°C. Two parts of these samples were subjected to DNA and RNA extraction for amplification of viral genomes. Another part was immersed in glutaraldehyde for the evaluation of fibrosis. Also, another part immersed in formaldehyde and subsequently embedded in paraffin, was (apart from diagnosis of DCM) not used in this study. Cryo-conserved parts of the samples that remained unused after the routine diagnostic tests were used for the immunohistochemical analyses described in this study.
Control group
Myocardial specimens from the left ventricle of 18 accident or suicide victims were embedded in TissueTek and snap-frozen in liquid nitrogen during autopsy and subsequently preserved at –80°C. In all subjects, no past history of cardiac or systemic disease was known. Hearts showed no macroscopic signs of structural abnormalities, ischaemia, or coronary lesions. The epidemiological characteristics of the control group are shown in Table 1.
Histology and immunohistochemistry
Acetone-fixed cryosections of biopsies from patients with DCM and control specimens were stained with antibodies specific for mDCs, pDCs, immature mDCs, mature mDCs, APCs, macrophages, T lymphocytes, endothelial progenitor cells (EPCs), as well as apoptotic cells (BCL-2+) using the catalysed signal amplification technique (CSA System™, Dako, Hamburg, Germany) as previously described.22 The sources, applications, and specificities of the antibodies used for immunohistochemical analyses are listed in Table 2. In addition to immunohistochemistry, apoptotic cells were also detected with the terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) method (Roche Diagnostics, Mannheim, Germany).
| Antibody | Clone | Against | Host | Ig type | Dilution | Source | Specificity |
|---|---|---|---|---|---|---|---|
| BCL-2 | 124 | Human | Mouse | IgG1 | 1:50 | DAKO | Apoptotic cells |
| CD11c | B-ly6 | Human | Mouse | IgG1 | 1:50 | BD Pharmingen | mDCs |
| CD3 | BW264/56 | Human | Mouse | IgG2a | 1:500 | Miltenyi Biotec | T cells |
| CD68 | PG-M1 | Human | Mouse | IgG3 | 1:500 | DAKO | Macrophages |
| CD69 | FN50 | Human | Mouse | IgG1 | 1:50 | Acris | Activated T cells |
| CD209 (DC-Sign) | DCN46 | Human | Mouse | IgG2b | 1:50 | BD Pharmingen | Immature mDCs |
| CD304 (BDCA-4/Neutropilin-1) | AD5-17F6 | Human | Mouse | IgG1 | 1:100 | Miltenyi Biotec | pDCs |
| CD34 | QBEnd 10 | Human | Mouse | IgG1 | 1:10 | DAKO | ECs, EPCs |
| Fascin | 55K-2 | Human | Mouse | IgG1 | 1:100 | DAKO | mDCs |
| HLA-DR | TAL.1B5 | Human | Mouse | IgG1 | 1:25 | DAKO | APCs |
| CCR7 (CD197) | E75 | Human | Rabbit | IgG1 | 1:100 | Epitomics | Migratory DCs |
| CD40 | HB14 | Human | Mouse | IgG1 | 1:50 | Miltenyi Biotec | APCs |
| CD83 | HB15e | Human | mouse | IgG1 | 1:50 | Serotec | Mature mDCs |
| GITR (CD357) | 621 | Human | Mouse | IgG1 | 1:100 | BioLegend | Regulatory T cells |
- a APCs, antigen-presenting cells; ECs, endothelial cells; EPCs, endothelial progenitor cells; mDCs, myeloid dendritic cells; pDCs, plasmacytoid dendritic cells.
For the purpose of co-localization of different markers for DCs and APCs, as well as for DC and apoptosis, we performed double staining of some of the cryosections with pairs of antibodies using a commercially available kit (EnVision™ G|2 Double Stain, DAKO, Hamburg, Germany).
Digital images of immunostained sections were taken with a CCD camera (Zeiss AxioCam HRc, Jena, Germany) at a magnification of ×100 (for double staining we used ×200 or ×400). For each analysis, the colour threshold for immunostained cells was manually adjusted in the images until the computerized detection matched the visual interpretation. Stained cells were counted digitally in a defined area of 0.2 mm2, using a digital image processing software (Image J 1.43u, Wayne Rasband, NIH, USA).
Assessment of tissue fibrosis
The EMB particles were immediately immersed and fixed in glutaraldehyde, subsequently post-fixed in osmium tetroxide (OSO4), dehydrated in ethanol, and embedded in Epon. Semi-thin sections (0.5 μm) were prepared and double stained with paraphenylenediamine and toluidine blue. The volume fraction of interstitial fibrosis was determined using light microscopy and stereologically quantified as previously described.5,23
Detection of the viral genome
DNA and RNA were extracted from frozen EMB samples. The viral genomes of parvovirus B19 (B19V), enterovirus (EV), and adenovirus (ADV) were detected using nested polymerase chain reaction (PCR) or reverse transcription–PCR (RT–PCR) as described elsewhere.24
Statistical analysis
Statistical analysis including descriptive, comparative, and relational analysis was performed using Sigma Plot Software Version 11.0 (Systat Software Inc.). The results are presented either as mean values ± standard deviation in the case of continuous data, or as median values with their respective 25th and 75th percentiles in the case of non-continuous data. Comparisons between groups were performed using the Mann–Whitney rank sum test. Correlation analyses were performed using the Spearman rank order test. For both tests, a P-value <0.05 was considered as statistically significant.
Results
Epidemiological and clinical data
There were no significant differences between the DCM and control groups in terms of age (Table 1). DCM patients had significantly higher body mass indexes and were, as expected, mostly males (both P = 0.04).
The majority of DCM patients were highly symptomatic, as two-thirds presented with dyspnoea of NYHA class III–IV, and had highly impaired and dilated LV (EF ∼30 ± 11%, LVEDD ∼62.5 ± 8 mm). Most DCM patients were treated with renin–angiotensin system (RAS) blocking agents (99%), beta-blockers (97%), diuretics, and aldosterone antagonists (78%), while a minority (8%) received cardiac glycosides.
In the studied time interval following the EMB (mean 4.7 ± 3.1 months), there was some amelioration of mean EF (ΔEF +8.8 ± 14.9%). Although such short-term improvement was, at least partly, due to HF therapy, another reason might be an overlapping myocarditis with rapid clinical improvement in some patients, as about one-third reported some recent history of infection.
Immunohistochemical and histological analysis
All examined DC subtypes were significantly lower in DCM biopsies compared with controls (see Table 3 and Figure 1). Such impairment among the myeloid subtypes was most severe in the case of fascin+ mDCs (2.7-fold, P < 0.001), followed by CD11c+ mDCs (1.7-fold, P = 0.33), then CD83+ mature mDCs (1.6-fold, P < 0.001), and CD209+ immature mDCs (1.2-fold, P = 0.034). PDCs as stained by CD304 were also significantly reduced (1.3-fold, P = 0.016), although not as severely as mDCs. Hence, a reduction of DCs was generally most strongly encountered in the (mature) myeloid subtype, suggesting a paucity of functionally active DCs in DCM myocardium. Double staining revealed co-localization of fascin with CD209, as well as fascin with CD83, while such an effect could not be observed between the above-mentioned mDC markers and CD304. These findings support our choice of antibodies, as they were thus proved to be specific for mDCs (fascin, CD83, and CD209) and pDCs (CD304), respectively.
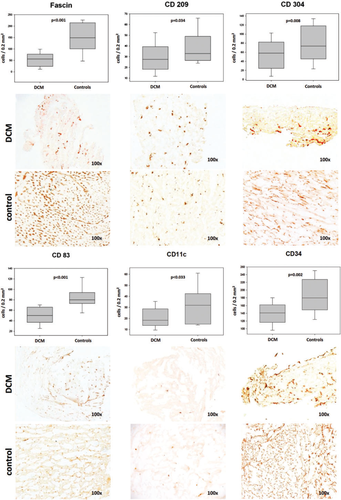
| Epitope (target cells): | Cells/0.2 mm2 area: | P-value | |
|---|---|---|---|
| DCM | Controls | ||
| Fascin (mDCs) | 56 (23.6, 77.4) | 149.3 (100.8, 214.8) | < 0.001 |
| CD11c (mDCs) | 18.5 (14, 29) | 32 (15, 43) | 0.033 |
| CD83 (mature mDCs) | 50 (37, 66) | 80 (73, 9) | < 0.001 |
| CD209 (immature mDCs) | 27.5 (18.3, 39.4) | 33 (26.5, 49) | 0.034 |
| CD304 (pDCs) | 58 (24.6, 82.5) | 73.5 (45.2, 118.3) | 0.016 |
| CD34 (ECs, EPCs) | 141 (116.5, 162) | 180 (149, 228) | 0.002 |
| HLA-DR (APCs) | 99.5 (76.8, 128.3) | 190 (156.4, 210) | < 0.001 |
| CD40 (APCs) | 53.5 (36.5, 69.5) | 84 (65, 106) | < 0.001 |
| CD3 (T cells) | 6.5 (4, 10) | 5 (3.8, 9.3) | 0.726 |
| CD69 (activated T cells) | 6 (3, 9.5) | 12.5 (5, 17.3) | 0.003 |
| CD68 (macrophages) | 14 (7, 26.4) | 14.5 (4.8, 30.3) | 0.830 |
| CCR7 (LN-bound migratory DCs) | 100.5 (73, 125) | 74 (35.8, 100.8) | 0.003 |
| GITR (Tregs) | 18 (10, 28) | 5.5 (2.8, 10) | < 0.001 |
| BCL-2 (apoptotic cells) | 93 (80, 118) | 17.5 (9, 33) | < 0.001 |
| TUNEL+ (apoptotic cells) | 214 (193, 235) | 23.5 (10, 54) | < 0.001 |
- a Data shown as median values (25th and 75th percentiles).
- b APCs, antigen-presenting cells; CCR7, C-C chemokine receptor 7; ECs, endothelial cells; EPCs, endothelial progenitor cells; LN, lymph node; mDCs, myeloid dendritic cells; pDCs, plasmacytoid dendritic cells; Treg, regulatory T cell; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labelling.
In order to assess DC activity, we used the markers HLA-DR and CD40. The human leucocyte antigen (HLA) DR is required for antigen presentation via the major histocompatibility complex and is generally used as a marker of professional APCs (i.e. DCs and macrophages). Its staining was, similarly to DCs, markedly reduced in DCM (1.9-fold, P < 0.001). The staining of sections with CD40 yielded similar results (1.6-fold reduction in DCM, P < 0.001). The similar results from the use of these markers, as well as their co-localization in some cells in double-stained sections (see Figure 2) is explained by the fact that the co-stimulatory protein CD40 is also found on APCs and is required for their activation. As the staining of macrophages with CD68 marker did not show any significant differences, it can be speculated that the reduction of CD40- and HLA- DR-positive cells is mainly due to a reduced antigen-presenting activity of DCs.
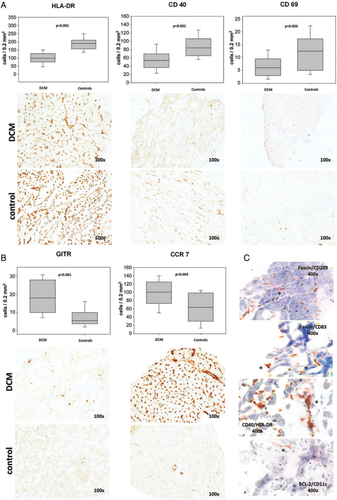
T lymphocytes stained with the CD3 epitope were not statistically different in the two groups. In order to differentiate T cells further and possibly to establish a link between T lymphocytes and DCs, we also used immunostaining with CD69. This marker is presented on T cells during activation by mature DCs. We found that the number of CD69+ cells was reduced in DCM biopsies, so that it might be assumed that the reduction of mature DCs is accompanied by a reduced activation of T cells. In contrast, Tregs stained with the GITR epitope were markedly enhanced in DCM (3.3-fold, P < 0.001).
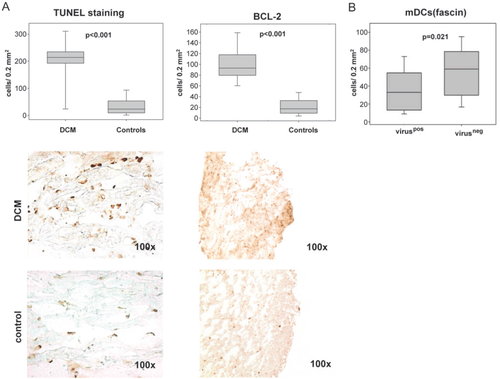
Both methods used for the detection of apoptosis (TUNEL reaction and immunostaining with BCL-2) revealed a marked increase in the DCM biopsy group (9.1- and 5.3-fold, P < 0.001 respectively, Figure 3). While many apoptotic cells had the appearance of DCs, we could also co-localize CD11c and BCL-2 using double staining in a subgroup of sections (Figure 2). These data imply that cellular death, including DCs, was increased in DCM hearts and could be (at least in part) responsible for the above-mentioned DC reduction. The CD34 staining of the EPCs, an indirect measure of capillary density, was markedly reduced in the DCM group (1.3.fold, P = 0.002) and correlated with the DC staining (see correlation below), thus providing the reduction in tissue vascularization as yet another possible explanation.
In order to find further possible explanations for the DC reduction, we also used immunostaining with the homing C-C chemokine receptor 7 (CCR7) and found it to be overexpressed in DCM compared with controls (1.4-fold, P = 0.003). Such up-regulation might suggest migration of DCs from myocardium to regional lymphoid tissue.
Subgroup analysis regarding virology
For the above-mentioned immunohistochemistry results, we compared virus-positive and -negative DCM subgroups. Testing for the presence of cardiotropic viruses in the myocardium was performed in 69% of DCM patients (n = 50), of whom 44% (n = 22) were found to be positive. The virus-positive and -negative subgroups did not differ in terms of EF or LVEDD [EF, P = 0.585; EF-FU (follow-up), P = 0.560; ΔEF, P = 0.162; LVEDD, P = 0.419; LVEDD-FU, P = 0.218; ΔLVEDD, P = 0.419]. We found significantly fewer mDCs in virus-positive compared with virus-negative EMBs (1.8-fold, P = 0.021). However, the subgroup analysis of all other used epitopes did not yield any significant differences (P > 0.05).
Correlation analyses
Fascin+ mDCs correlated with LV function improvement during the follow-up period (ΔEF, r = 0.303, P = 0.033). CD83+ mature mDCs also correlated with ΔEF (r = 0.376, P = 0.04). For pDCs, we found an inverse correlation with LVEDD at follow-up (LVEDD-FU, r = –0.322, P = 0.026). Hence, we saw several statistical links between DCs in the DCM biopsies and LV function and size improvement during the course of disease. CD3+ T cells inversely correlated with ΔEF (r = –0.293, P = 0.048) and positively correlated with ΔLVEDD (r = 0.331, P = 0.036). HLA-DR expression did not correlate significantly with ΔEF (P > 0.05), but with the EF-FU (r = 0.324, P = 0.023). CCR7 was strongly correlated to the EF-FU (r = 0.498, P = 0.004) and inversely with the LVEDD-FU (r = -0.529, P = 0.004) (Figure 4). We found no significant correlations (P > 0.05) between echocardiographic parameters and immature mDCs, CD40+ cells, macrophages, or Tregs. These data suggest an expected negative effect of inflammatory T cells on the course of DCM. Unexpectedly, however, DCs seem to have an opposite (protective) effect, as they correlated with LV size and function improvement.
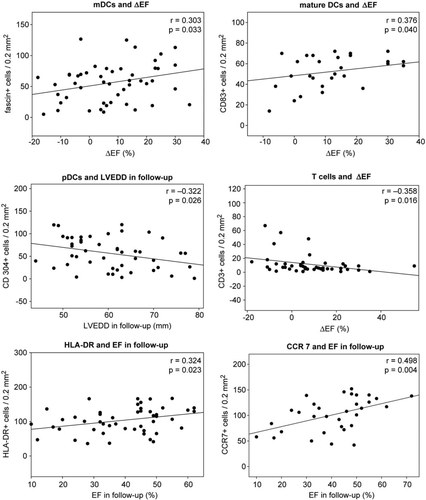
In line with the above findings, we saw a significant inverse correlation of tissue fibrosis with DCs and DC activity. mDCs (r = –0.258, P = 0.049), pDCs (r = –0.272, P = 0.034), and HLA-DR+ cells (r = –0.335, P = 0.009) were inversely correlated with fibrosis (Figure 5). A similar correlation was seen for activated T cells (r = –0.275, P = 0.033). Therefore, we observe a possible correlation between myocardial tissue damage and reduction of mature DCs and their activation.
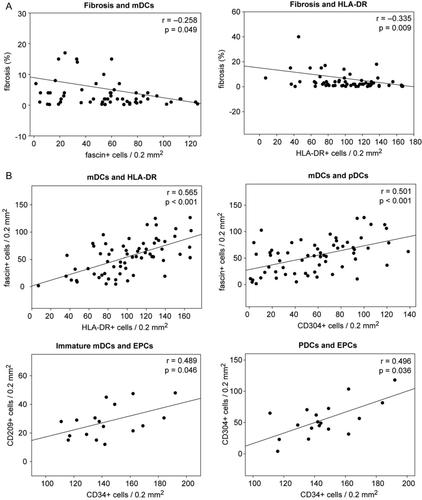
As expected, fibrosis inversely correlated with EF at the time of biopsy (r = –0.278, P = 0.022), but even more so with EF, LVEDD, and ΔEF at follow-up (r = –0.46, P < 0.001; r = 0.34, P = 0.013; and r = 0.282, P = 0.047, respectively).
Correlation analyses among immune cells showed strong positive correlations between mDCs, pDCs, immature DCs, and HLA-DR. Immature mDCs (CD209+) and pDCs (CD304+) correlated strongly with EPCs (CD34; r = 0.489, P = 0.046 and r = 0.296, P = 0.036, respectively) (Figure 5). While fascin+ mDCs also correlated with activated T cells (r = 0.325, P = 0.012), no such correlations were found for pDCs. This finding is in line with the belief that while mDCs are responsible for T cell activation, pDCs have rather tolerogenic properties. No significant correlations were found between DCs and CD3+ cells or macrophages.
Discussion
This is the first quantitative analysis of the presence of DCs in the myocardium of patients with DCM. These immune cells were identified using established immunohistochemical techniques22,25,26 and were correlated to histological and echocardiographic parameters. Beyond DCs, we additionally investigated the presence of T lymphocytes and macrophages. Given the obvious difficulty in obtaining myocardial biopsies from healthy individuals, we used post-mortem specimens from aged-matched accident and suicide victims as controls.
The DCs of different subtypes and maturation stages were significantly reduced in EMBs of DCM hearts. Similarly, markers of antigen presentation, such as HLA-DR and CD40, and of T cell activation were also reduced. In contrast, Tregs were increased in DCM, while macrophages did not differ significantly compared with controls. Hence, there was not a general trend for a reduction of immune cells in DCM biopsies, but rather a specific reduction of DCs and other cells which related to their activity.
The two studied subtypes of DCs (myeloid and plasmacytoid) have been attributed different and in part opposing functions regarding their interactions with T cells. mDCs are known key inflammatory T-cell activators in adaptive immunity. Following antigen contact, they maturate in regional lymph nodes, and induce T-cell differentiation and proliferation. Such an inflammatory role for mDCs has also been described in myocardial tissue following acute myocardial infarction, as they seem to contribute greatly to T-cell activation that leads to tissue remodelling and deterioration of ventricular function.27 While circulatory DC precursors, as well as other immune cells are reduced in peripheral blood of humans in the event of an acute myocardial infarction,28,29 our study group recently found out that such peripheral reduction was due to an increased infiltration of DCs, T cells, and macrophages in human infarcted myocardial tissue.30 Therefore, our present finding of reduction of myocardial DCs and T-cell activation in DCM seemed at first unexpected. The overexpression of Tregs and CCR7, the observed increased cellular death, the decrease in endothelial cell expression, as well as the inverse correlation of DCs with tissue fibrosis provided us with several possible explanations for our results.
-
Unlike mDCs, pDCs have been described as having anti-inflammatory properties in maintaining adaptive immune tolerance through activation of Tregs. The interaction of Tregs with pDCs suppresses the proliferation and activation of inflammatory T cells.12 We used the glucocorticoid-induced tumour necrosis factor (TNF) receptor-related gene (GITR) for the staining of Tregs, as this marker is known to be mainly expressed in these cells.31 Although the increased presence of GITR would be suggestive of a higher presence of Tregs in DCM biopsies, we are aware of the limited specificity of this marker for Tregs. Indeed, GITR is also known to be expressed, to a lesser extent, in all other types of T cells; hence, we cannot exclude the possibility that other GITR-expressing cells could be responsible for the observed overexpression.
-
Another possible mechanism that would explain our present findings is cellular death, due to myocardial tissue damage. Apoptotic DCs can induce the proliferation of Tregs and inhibit DC maturation and T-cell activation, as was shown in murine in vitro and in vivo models.32 In our study, tissue fibrosis, being an indicator of myocardial tissue damage, was inversely correlated with mDCs, thus providing a link between myocardial tissue damage and the depletion of mature DCs. As we evaluated cellular apoptosis, we found it indeed markedly increased in DCM biopsies in using both detection techniques (TUNEL and immunohistochemistry) and could co-localize mDCs with the apoptosis marker BCL-2.
-
The reduced presence of DCs might also result from an insufficient recruitment into fibrotic myocardium, due to insufficient tissue vascularization. Supportive of this suggestion is the underexpression of the endothelial marker CD34 in our DCM myocardial tissues, as well as its strong positive correlation with the markers for immature mDCs and pDCs.
-
The C-C chemokine receptor 7 is known to be mainly involved in migration and homing of DCs into regional lymphoid tissue.33 CCR7 was overexpressed in the biopsies of our DCM patients and could well be the reason for the reduced presence of DCs in these biopsies, as DCs could have migrated in regional lymph nodes. For obvious ethical reasons, we could not examine lymphoid tissue from our patients, as EMBs were performed primarily for diagnostic reasons.
The specific role of DCs in human myocarditis and DCM is unknown as yet. It has been suggested that the activation of T cells by DCs and the following inflammatory processes lead to increased myocardial damage through fibrosis and remodelling.34 Our present observations do not support such a hypothesis. On the contrary, our results show that the reduction of DCs in myocardium of DCM patients is associated with a worse disease outcome in terms of LV function and fibrosis. Our findings are supported by new evidence, showing that an increased proliferation of DCs does not lead to DCM in a murine model of experimental myocarditis.35
Besides the existing doubts that autoimmune mechanisms following viral infection lead to DCM in humans, and the lack of convincing results in experience with immune modulation therapy, viral presence and its persistence in the myocardium of patients with DCM have been associated with unfavourable disease outcome and progression of cardiac dysfunction in terms of EF.36 Viral clearance, on the other hand, was associated with an improvement of LV function and size when treated with interferon-beta.37 In our study, we saw an association between myocardial mDC reduction and viral presence, and a correlation of mDC reduction with unfavourable short-term outcome of DCM (in terms of EF, LVEDD, and fibrosis). Thus, it could be possible that DCs have protective properties in DCM, due to their antiviral activity. The discrepancy of the hypothesis drawn from previous studies on EAM and our present work could be explained by different roles for DCs in different types of myocarditis (viral vs. EAM). DCs are central in autoimmune inflammation—they might therefore play a key role in such processes in EAM. On the other hand, their antigen-presenting function might be essential in triggering an antiviral immune response following viral myocarditis with the positive goal of virus elimination, thus leading to recovery or better prognosis.
Our DCM patients were highly symptomatic and had already developed clinical characteristics of DCM, such as LV dilation and function impairment. Although according to our medical data about one-third had a previous history of infection, they did not show clinical signs of myocarditis at the time of EMB. As viral myocarditis often resolves spontaneously, in such benign cases there is no medical indication for EMB; thus such individuals were not represented in our study group. It is therefore possible that the results would have been quite different in a hypothetical study collective of myocarditis with spontaneous recovery. Any possible conclusions from this study may therefore not be applicable to cell-mediated inflammatory processes in mild forms of myocarditis with spontaneous recovery or initial stages of DCM. Follow-up biopsies were also not available, as there is usually no medical indication or patient benefit for the repetition of such an invasive procedure. At the time of publication, we do not have sufficient data on survival. Our comments on prognosis are at this stage limited to short-term echocardiographic parameters.
In conclusion, DCs as characterized by immunostaining were less abundant in myocardium of DCM patients with chronic HF compared with controls, possibly due to apoptosis and insufficient vascularization in the damaged myocardium and/or immunomodulating mechanisms led by regulatory T cells. As such a reduction of mDCs was more marked in DCM hearts tested positive for cardiotropic viruses, we assume that there is a connection between these cells and myocardial virus clearance. The immunohistochemistry of DCs in EMBs of DCM could prove to be useful as a prognostic marker in terms of disease progression. We are aware of the fact that further prospective and functional experimental studies are necessary to confirm our observations and underline any possible conclusions drawn from this study.
Funding
The Interdisciplinary Center for Clinical Research of the University Hospital of Jena.
Conflict of interest: none declared.




