Adaptive servo-ventilation therapy improves cardiac sympathetic nerve activity in patients with heart failure
Abstract
Aims
This study investigated whether abnormal cardiac sympathetic nerve activity (SNA) is associated with the severity of central sleep apnoea (CSA) and whether adaptive servo-ventilation (ASV) therapy can improve cardiac SNA in heart failure (HF) patients with predominant CSA.
Methods and results
Overnight polysomnography was conducted to diagnose CSA. Cardiac SNA was analysed by [123I]metaiodobenzylguanidine scintigraphy in 26 consecutive HF patients with predominant CSA. Of the 26 patients, 10 agreed to ASV therapy. Cardiac SNA was analysed 6 months after initiating ASV based on a non-randomized protocol. The apnoea–hypopnoea index and central apnoea index were significantly correlated with the washout rate (WR) and a delayed heart to mediastinal (H/M) ratio, suggesting that SNA is associated with abnormal breathing patterns. The WR, H/M ratio, plasma BNP level, and LVEF were significantly improved (WR, 40.0 ± 11.6% vs. 34.6 ± 11.4%, P = 0.046; H/M ratio, 1.5 ± 0.1 vs.1.8 ± 0.3, P = 0.013; ln BNP, 5.4 ± 1.0 vs. 4.6 ± 1.2, P = 0.007; and LVEF, 43.8 ± 10.4% vs. 47.0 ± 10.6%, P < 0.001) in the ASV group patients, but not in the non-ASV group patients. Multiple linear regression analyses showed that a decreased WR was strongly associated with an increased LVEF (coefficient = –0.454, P = 0.013).
Conclusions
Abnormal cardiac SNA could be significantly correlated with the severity of CSA in HF patients. ASV therapy might improve cardiac function in these patients by partially mediating cardiac SNA regulation.
Introduction
Despite the development of therapeutic management for heart failure (HF), the mortality rate of HF patients remains high.1 Because elevated sympathetic nerve activity (SNA) is associated with a poor prognosis in HF patients,2 inhibiting the elevated SNA in the failing heart seems to be a logical target for HF therapy.
Sleep-disordered breathing (SDB), particularly periodic breathing patterns such as repeated central sleep apnoea (CSA) and hypopnoea, is frequently encountered in HF patients,3,4 and is associated with increased mortality and cardiac events.5,6 Hypocapnoea and circulation delays with elevated central chemoreceptor sensitivity are known to contribute to abnormal breathing during sleep in HF patients.7 Decreased lung inflation and hypoxic stress due to abnormal breathing could enhance cardiac SNA.8,9
Adaptive servo-ventilation (ASV) therapy was originally developed to eliminate SDB, including CSA.10 The ASV device adjusts its setting according to breathing effort, and it maintains a steady minute ventilation and low-grade positive end-expiratory pressure. A very recent study failed to observe strong haemodynamic disadvantages during CSA.11,12 However, we hypothesized that these beneficial effects of ASV therapy would reduce the elevated SNA caused by CSA and prevent short- or long-term haemodynamic disadvantages in HF patients by inhibiting elevated systemic vascular resistance. Previous studies showed improved cardiac contractile function in HF patients with CSA after several months of ASV therapy.3,13 Moreover, recent studies have shown that ASV therapy improved the short-term prognosis of HF patients.14,15 However, it has not been fully evaluated whether cardiac SNA is associated with the severity of CSA in patients with HF, and whether ASV therapy for CSA can improve cardiac SNA in patients with CSA. This study was designed to clarify these points.
Methods
Study protocol
We enrolled 35 consecutive HF patients seen between April 2010 and March 2011, who had not been admitted to the hospital due to worsening HF in the 6 months before initiating ASV therapy, whose LVEF was <55%, and whose NYHA functional class was II or III, as previously reported.16 The protocol is summarized in Figure 1. Briefly, overnight polysomnography (PSG) was conducted after admission to the Akita University Hospital Sleep Center. Strong evidence indicates that CSA is closely associated with mortality and a poor prognosis in HF patients.5,6 Twenty-six HF patients experiencing predominant CSA events were enrolled in this study; nine HF patients were excluded because their apnoea–hypopnoea index (AHI) scores were <5 or they had predominant obstructive apnoea events. The patients were divided into two groups: those with an AHI score ≥5 and <15 (group A, n = 7), and those with an AHI score ≥15 (group B, n = 19). Of the group B patients, 10 agreed to ASV (Autoset CS®; ResMed, Sydney, Australia) therapy (ASV group). [123I]Metaiodobenzylguanidine (MIBG) myocardial scintigraphy and echocardiography were performed before and 6 months after ASV treatment. Conventional blood samples to determine the plasma BNP levels were obtained before and 6 months after ASV therapy. Each patient's blood pressure (BP) and heart rate (HR) were recorded three times in the supine position and averaged before initiating ASV therapy and at each visit to the outpatient clinic. Patients who had recovered from acute or decompensated HF, and who could not continue to take their drugs for their underlying disease were excluded from the study. If the patients experienced a decrease in BP (systolic BP <100 mmHg) or increase in BP (systolic BP >140 mmHg), calcium antagonists or diuretics were decreased or added by half-increments. In addition, for those HF patients who experienced a minimum decrease or increase of 3 kg in body weight, diuretics were decreased or added, respectively, by half-increments.17 Due to their cardioprotective effects, the doses of ACE inhibitors, ARBs, or beta-blockers were not changed during the follow-up visits. We examined the relationship between MIBG scintigraphic parameters and severity of CSA or changes in HF parameters in those CSA patients who agreed or did not agree to ASV therapy (Figure 1).

All patients provided written informed consent. The study protocol was approved by the clinical research and ethics committee of the University of Akita.
Overnight polysomnography
All patients underwent complete overnight PSG using the ProFusion PSG® Sleep Diagnostic system (Compumedics, Victoria, Australia), which continuously monitors the electroencephalogram, electro-oculogram (used in sleep staging), oxygen saturation (SaO2), airflow, snoring, and thoracoabdominal motion. Apnoea was defined as an absence of inspiration without ribcage and abdominal motion for ≥10 s; hypopnoea was defined as a ≥30% reduction in monitored airflow accompanied by a ≥4% decrease in SaO2. Arousal responses were defined according to the recommendations of the American Sleep Disorders Association. The AHI was defined as the number of episodes of apnoea and hypopneoa per hour of sleep. Patients with an AHI score ≥5/h were diagnosed with SDB. A diagnosis of predominant CSA was assigned to those patients with an AHI score ≥5/h when ≥50% of the events were labelled as central rather than obstructive apnoea. Those HF patients with predominant CSA were divided into two groups: A, AHI <15/h, and B, AHI ≥15/h.
[123I]Metaiodobenzylguanidine myocardial scintigraphy
All patients were injected intravenously with a dose of 111 MBq of MIBG (Daiichi Radioisotope Laboratories, Tokyo, Japan) after fasting and resting. A 10 min static acquisition of the chest anterior view was obtained using a three-headed gamma camera with a low-energy, parallel hole collimator (MULTISPECT 3; Siemens Medical Solutions, Erlangen, Germany) at 5 min (early imaging) and 4 h (delayed imaging) after injection. Data processing was performed using a data processing system (ICON; Siemens Medical Solutions). No attenuation or decay corrections for the half-life of 123I were used. The LV MIBG activity was measured within a manually drawn region of interest (ROI) around the heart. A 10 × 10 pixel ROI was placed over the upper mediastinal area, and the heart to mediastinal (H/M) ratio at early and delayed imaging were calculated from the mean counts per pixel. The H/M ratio and washout rate (WR) were calculated from the average counts in each ROI by two independent observers. The WR of MIBG in the myocardium was determined as a percentage of the rate of decrease in myocardial counts over time between early and delayed imaging. To evaluate cardiac SNA in the enrolled HF patients, we used both the WR and delayed H/M ratio measurements acquired by MIBG scintigraphy.
Echocardiography
Two-dimensional, M-mode, and Doppler echocardiography (iE33; Philips Medical Systems, Andover, MA, USA) were performed to evaluate various cardiac function parameters in the patients. The LVEF was determined from an apical four-chamber view using Simpson's method. The sonographers were blinded to the PSG results and were not involved in treating the patients.
Adaptive servo-ventilation therapy initiation
Adaptive servo-ventilation therapy was initiated for 19 consecutive HF patients with CSA. The HF patients were fitted with an ASV mask, and it was determined whether they would receive ASV treatment during the initial 3 days of the treatment period, based on our previous study.3 During the first 20 min of ASV treatment, each patient's HR, SaO2, and BP were monitored every 5 min. Some patients refused ASV therapy due to the positive airway pressure or mask discomfort. Consequently, 10 HF patients agreed (ASV group) while nine patients did not agree (non-ASV group) to ASV therapy. A follow-up assessment was conducted 6 months later. ASV therapy was initiated by experienced physicians who were familiar with the ASV device. An expiratory positive airway pressure of 5 cmH2O and inspiratory pressure support between 3 and 10 cmH2O were used. The ASV-treated patients were defined as those whose device usage was >4 h per night during the follow-up period. Compliance data were downloaded from the ASV devices and checked monthly at the outpatient clinic.
Statistical analysis
Continuous variables are expressed as the mean ±standard deviation (SD). Student's t-test was used to compare groups of continuous and normally distributed data, whereas the Mann–Whitney U-test was used for non-normally distributed data. Correlations were analysed using Pearson's correlation coefficient. Plasma BNP levels were natural log-transformed. Multiple linear regression analyses, using several parameters with a significance of <0.10 by univariate analysis, were performed to estimate factors influencing the changes in LVEF. A P-value <0.05 was considered statistically significant. All analyses were performed using SAS software version 8.0 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics and sleep study results
The baseline characteristics of the enrolled HF patients are summarized in Table 1. No significant differences in age, sex ratio, body mass index, body weight, medical history, BP, HR, pharmacological data, LVEF, or estimated glomerular filtration rate (eGFR) were found between the two groups. Sleep parameters, including the AHI score and arousal index, were higher in group B than in group A. The mean SaO2 was lower in group B than in group A. The WR was significantly elevated in group A compared with group B. An elevated H/M ratio was found in group A compared with group B. The plasma BNP and high-sensitive C-reactive peptide levels were higher in group B than in group A. The baseline data of the ASV-treated and non-ASV-treated patients are summarized in Table 2. There were no significant differences between the two groups in each parameter.
| Group A (n = 7) | Group B (n = 19) | P-value | |
|---|---|---|---|
| Age, years | 63.4 ± 8.3 | 63.2 ± 14.2 | 0.795 |
| Male sex, n (%) | 6 (85.7) | 15 (79.0) | 0.703 |
| BMI, kg/m2 | 23.0 ± 3.7 | 25.9 ± 5.9 | 0.235 |
| Body weight, kg | 62.5 ± 8.6 | 70.4 ± 17.3 | 0.355 |
| Hypertension, n (%) | 5 (71.4) | 12 (63.2) | 0.694 |
| Diabetes mellitus, n (%) | 4 (57.1) | 9 (47.4) | 0.658 |
| Hyperlipidaemia, n (%) | 2 (28.6) | 11 (57.9) | 0.185 |
| CAD, n (%) | 2 (28.6) | 6 (31.6) | 0.883 |
| Valvular heart disease, n (%) | 4 (57.1) | 8 (42.1) | 0.495 |
| Cardiomyopathy, n (%) | 2 (28.6) | 5 (26.3) | 0.908 |
| Blood pressure | |||
| Systolic, mmHg | 110.6 ± 16.5 | 121.5 ± 24.1 | 0.236 |
| Diastolic, mmHg | 74.6 ± 15.8 | 73.2 ± 13.1 | 0.839 |
| Heart rate, b.p.m. | 76.8 ± 8.9 | 77.4 ± 11.1 | 0.931 |
| Medication, n (%) | |||
| ACEIs/ARBs | 6 (85.7) | 18 (94.7) | 0.444 |
| Beta-blockers | 7 (100.0) | 14 (100) | 1.000 |
| Calcium antagonists | 2 (28.6) | 3 (15.8) | 0.463 |
| Statins | 3 (42.9) | 11 (57.9) | 0.495 |
| Aldosterone angatonists | 6 (85.7) | 17 (89.5) | 0.790 |
| Diuretics | 6 (85.7) | 19 (100.0) | 0.093 |
| Polysomnography data | |||
| AHI, n/h | 11.5 ± 5.3 | 37.7 ± 11.6 | <0.001 |
| AI, n/h | 3.3 ± 3.7 | 20.2 ± 10.1 | <0.001 |
| Obstructive type, n/h | 0.6 ± 1.1 | 3.6 ± 4.9 | 0.053 |
| Central type, n/h | 2.7 ± 3.2 | 16.5 ± 9.6 | <0.001 |
| HI, n/h | 8.1 ± 4.5 | 17.4 ± 9.7 | 0.046 |
| Mean SaO2, % | 96.7 ± 1.4 | 94.8 ± 2.3 | 0.029 |
| Arousal index, n/h | 19.2 ± 17.0 | 33.4 ± 18.2 | 0.006 |
| Total sleep time, h | 5.4 ± 0.8 | 5.5 ± 0.9 | 0.664 |
| Echocardiographic parameters | |||
| LVEF, % | 43.2 ± 15.7 | 42.3 ± 12.7 | 0.581 |
| LVEDV, mL | 174.0 ± 40.8 | 191.8 ± 43.1 | 0.247 |
| LVESV, mL | 104.4 ± 44.4 | 113.8 ± 48.2 | 0.524 |
| [123I]MIBG data | |||
| Washout rate, % | 29.5 ± 9.1 | 42.2 ± 12.9 | 0.019 |
| H/M ratio (early) | 2.46 ± 0.31 | 1.76 ± 0.24 | <0.001 |
| H/M ratio (delay) | 2.21 ± 0.31 | 1.62 ± 0.24 | <0.001 |
| Laboratory data | |||
| eGFR, mL/min/1.73 m2 | 62.4 ± 14.3 | 47.8 ± 12.8 | 0.034 |
| hs-CRP levels, mg/dL | 0.08 ± 0.04 | 0.25 ± 0.21 | 0.004 |
| BNP levels, pg/mL | 111.3 ± 72.2 | 475.9 ± 539.6 | 0.040 |
- a Values are reported as mean ± standard deviation, or n (%) where indicated.
- b AHI, apnoea–hypopnoea index; AI, apnoea index; BMI, body mass index; eGFR, estimated glomerular filtration rate; HI, hypopnoea index, H/M ratio, heart to mediastinum ratio; hs-CRP, high-sensitive C-reactive protein; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; [123I]MIBG, [123I]metaiodobenzylguanidine; SaO2, oxygen saturation.
| ASV group (n = 10) | Non-ASV group (n = 9) | P-value | |
|---|---|---|---|
| Age, years | 61.3 ± 15.2 | 65.2 ± 13.6 | 0.683 |
| Male sex, n (%) | 7 (70.0) | 8 (88.9) | 0.313 |
| BMI, kg/m2 | 27.2 ± 6.3 | 24.5 ± 5.5 | 0.594 |
| Body weight, kg | 73.4 ± 18.7 | 67.1 ± 16.0 | 0.513 |
| Hypertension, n (%) | 7 (70.0) | 5 (55.6) | 0.515 |
| Diabetes mellitus, n (%) | 5 (50.0) | 4 (44.4) | 0.809 |
| Dyslipidaemia, n (%) | 7 (70.0) | 4 (44.4) | 0.343 |
| Coronary artery disease, n (%) | 3 (30.0) | 3 (33.3) | 0.876 |
| Valvular heart disease, n (%) | 3 (30.0) | 5 (55.6) | 0.260 |
| Cardiomyopathy, n (%) | 2 (20.0) | 3 (33.3) | 0.509 |
| Blood pressure | |||
| Systolic, mmHg | 116.5 ± 19.1 | 127.0 ± 28.9 | 0.191 |
| Diastolic, mmHg | 69.8 ± 8.4 | 76.9 ± 16.6 | 0.251 |
| Heart rate, b.p.m. | 72.6 ± 7.1 | 78.0 ± 11.9 | 0.622 |
| Medication, n (%) | |||
| ACEIs/ARBs | 9 (90.0) | 9 (100.0) | 0.329 |
| Beta-blockers | 10 (100.0) | 9 (100.0) | 1.000 |
| Calcium antagonists | 1 (10.0) | 2 (22.2) | 0.466 |
| Statins | 6 (60.0) | 5 (55.6) | 0.845 |
| Aldosterone antagonists | 9 (90.0) | 8 (88.9) | 0.937 |
| Diuretics | 9 (90.0) | 8 (88.9) | 0.937 |
| Polysomnography data | |||
| AHI, n/h | 35.7 ± 13.7 | 39.9 ± 8.9 | 0.369 |
| AI, n/h | 18.2 ± 11.3 | 22.4 ± 8.7 | 0.540 |
| Obstructive, n/h | 3.2 ± 5.6 | 4.0 ± 4.2 | 0.651 |
| Central, n/h | 15.1 ± 9.3 | 18.1 ± 10.2 | 0.513 |
| HI, n/h | 17.4 ± 11.4 | 17.5 ± 8.2 | 0.935 |
| Mean SaO2, % | 95.5 ± 1.3 | 94.2 ± 3.0 | 0.429 |
| Arousal index, n/h | 31.4 ± 17.0 | 35.6 ± 20.2 | 0.683 |
| Total sleep time, h | 5.7 ± 0.7 | 5.4 ± 1.0 | 0.142 |
| Echocardiographic parameters | |||
| LVEF, % | 43.8 ± 10.4 | 40.6 ± 15.3 | 0.682 |
| LVEDV, mL | 197.3 ± 49.7 | 185.7 ± 36.4 | 0.713 |
| LVESV, mL | 113.5 ± 47.9 | 114.2 ± 51.6 | 0.978 |
| [123I]MIBG data | |||
| Washout rate, % | 40.0 ± 11.6 | 44.5 ± 14.5 | 0.513 |
| H/M ratio (early) | 1.71 ± 0.17 | 1.81 ± 0.29 | 0.327 |
| H/M ratio (delay) | 1.70 ± 0.30 | 1.55 ± 0.14 | 0.366 |
| Laboratory data | |||
| eGFR, mL/min/1.73 m2 | 48.2 ± 12.1 | 47.5 ± 14.3 | 0.807 |
| hs-CRP levels, mg/dL | 0.19 ± 0.13 | 0.31 ± 0.27 | 0.413 |
| BNP levels, pg/mL | 317.2 ± 243.2 | 652.3 ± 722.5 | 0.683 |
- a Values are reported as mean ± standard deviation, or n (%) where indicated.
- b AHI, apnoea–hypopnoea index; AI, apnoea index; ASV, adaptive servo-ventilation; BMI, body mass index; eGFR, estimated glomerular filtration rate; HI, hypopnoea index, H/M ratio, heart to mediastinum ratio; hs-CRP, high-sensitive C-reactive protein; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; [123I]MIBG, [123I]metaiodobenzylguanidine; SaO2, oxygen saturation.
Relationship between scintigraphic parameters and central sleep apnoea severity in heart failure patients
Table 3 shows the relationship between the scintigraphic parameters and PSG data in the 26 HF patients. The AHI was significantly correlated with the WR and H/M ratio. Additionally, the central apnoea index was significantly correlated with the WR and H/M ratio. The apnoea index was significantly correlated with the H/M ratio. Significant correlations were shown between the hypopnoea index and the WR. No significant correlations between the obstructive apnoea index and the WR or H/M ratio were noted.
| Washout rate | H/M ratio | |||||
|---|---|---|---|---|---|---|
| r | R2 | P-value | r | R2 | P-value | |
| AHI | 0.391 | 0.208 | 0.019 | –0.477 | 0.227 | 0.014 |
| AI | 0.290 | 0.067 | ns | –0.405 | 0.164 | 0.040 |
| HI | 0.611 | 0.190 | 0.026 | –0.286 | 0.082 | 0.047 |
| Central | 0.462 | 0.134 | 0.048 | –0.474 | 0.225 | 0.014 |
| Obstructive | 0.565 | 0.035 | ns | –0.049 | 0.002 | NS |
- a AHI, apnoea–hypopnoea index; AI, apnoea index; HI, hypopnoea index; H/M ratio, heart to mediastinum ratio; NS, non-significant.
Patient parameters 6 months after adaptive servo-ventilation therapy
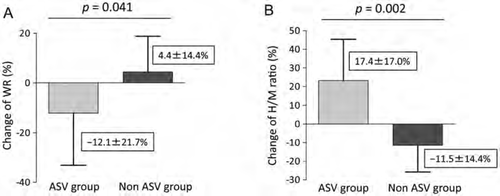
After the mask-fitting period, four patients discontinued ASV and five patients could not use the ASV device for more than 4 h per night; these were the non-ASV-treated patients. These HF patients did not experience decreased cardiac function or other disadvantages during the follow-up period. During the follow-up period, two HF patients required a decrease in diuretic dose and one patient required a decrease in calcium antagonist dose among the ASV-treated patients, whereas the drug administration was not changed in the non- ASV-treated patients. As shown in Figure 2, the relative changes in WR and H/M ratio were significantly higher in the ASV group when compared with the non-ASV group patients [WR (ASV group) –12.1 ±21.7% vs. (non-ASV group) 4.4 ±14.4%, P = 0.041, Figure 2A; H/M ratio (ASV group) 17.4 ±17.0% vs. (non-ASV group) –11.5 ±14.4%, P = 0.002, Figure 2B, respectively]. Table 4 shows the changes in physical findings and in the echocardiographic, laboratory, and PSG data of the HF patients with or without ASV therapy. The LVEF and mean SaO2 increased, whereas the LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), plasma BNP level, AHI, central apnoea index, hypopnoea index, and arousal index decreased after 6 months in the ASV group, but not in the non-ASV group. The average ASV use time was greater in ASV-treated patients than in the non-ASV-treated patients (6.2 ±0.6 vs. 0.4 ±0.5 h, respectively P < 0.001). Cardiac function and plasma BNP levels did not change in the HF patients who used ASV therapy for <4 h (LVEF, 45.8 ±14.7 to 44.6 ±13.3, P = 0.133; ln BNP, 5.3 ±1.5 to 5.2 ±1.8, P = 0.795) when compared with those of the ASV group during the follow-up period (LVEF, 43.8 ±10.4 to 47.0 ±10.6, P < 0.001; ln BNP, 5.4 ±1.0 to 4.6 ±1.2, P = 0.010). Stepwise multiple linear regression analyses, including the relative change in WR, delayed H/M ratio, and AHI score, showed that the relative change in WR was the strongest parameter predicting LVEF improvement (coefficient = –0.454, standard error = 0.094, P = 0.013).
| ASV group (n = 10) | Non-ASV group (n = 9) | |||||
|---|---|---|---|---|---|---|
| Before | After 6 months | P-value | Before | After 6 months | P-value | |
| Physical findings | ||||||
| Heart rate, b.p.m. | 72.6 ± 7.1 | 72.5 ± 4.8 | NS | 78.0 ± 11.9 | 79.1 ± 10.3 | NS |
| Blood pressure | ||||||
| Systolic, mmHg | 116.5 ± 19.1 | 112.8 ± 12.4 | NS | 127.0 ± 28.9 | 125.8 ± 26.5 | NS |
| Diastolic, mmHg | 69.8 ± 8.4 | 69.0 ± 6.9 | NS | 76.9 ± 16.6 | 75.8 ± 12.7 | NS |
| BMI, kg/m2 | 27.2 ± 6.3 | 27.8 ± 5.6 | NS | 24.5 ± 5.5 | 24.9 ± 5.7 | NS |
| Body weight, kg | 73.4 ± 18.7 | 75.0 ± 17.2 | NS | 67.1 ± 16.0 | 68.0 ± 16.7 | NS |
| Echocardiographic data | ||||||
| LVEF, % | 43.8 ± 10.4 | 47.0 ± 10.6 | <0.001 | 40.6 ± 15.3 | 40.2 ± 14.2 | NS |
| LVEDV, mL | 197.3 ± 49.7 | 182.2 ± 50.9 | 0.004 | 185.7 ± 36.4 | 187.8 ± 28.8 | NS |
| LVESV, mL | 113.5 ± 47.9 | 91.0 ± 30.5 | 0.045 | 114.2 ± 51.6 | 117.9 ± 44.9 | NS |
| Laboratory data | ||||||
| Ln BNP | 5.4 ± 1.0 | 4.6 ± 1.2 | 0.010 | 5.7 ± 1.5 | 5.8 ± 1.6 | NS |
| Polysomnography data | ||||||
| AHI, n/h | 35.7 ± 13.7 | 9.0 ± 4.9 | <0.001 | 39.9 ± 8.9 | 37.2 ± 9.7 | NS |
| AI, n/h | 18.2 ± 11.3 | 4.2 ± 6.5 | <0.001 | 22.4 ± 8.7 | 21.7 ± 9.3 | NS |
| Obstructive, n/h | 3.2 ± 5.6 | 3.3 ± 5.9 | NS | 4.0 ± 4.2 | 7.3 ± 3.2 | NS |
| Central, n/h | 15.1 ± 9.3 | 0.9 ± 1.1 | <0.001 | 18.1 ± 10.2 | 13.7 ± 6.3 | NS |
| HI, n/h | 17.4 ± 11.4 | 7.4 ± 3.5 | <0.001 | 17.5 ± 8.2 | 15.4 ± 6.6 | NS |
| Mean SaO2, % | 95.5 ± 1.3 | 97.3 ± 0.8 | 0.002 | 94.2 ± 3.0 | 94.4 ± 3.1 | NS |
| Arousal index, n/h | 31.4 ± 17.0 | 16.9 ± 9.9 | <0.001 | 35.6 ± 20.2 | 35.3 ± 15.2 | NS |
| Total sleep time, h | 5.71 ± 0.7 | 5.53 ± 1.0 | NS | 5.4 ± 1.0 | 5.5 ± 0.6 | NS |
- a AHI, apnoea–hypopnoea index; AI, apnoea index; ASV, adaptive servo-ventilation; BMI, body mass index; eGFR, estimated glomerular filtration rate; HI, hypopnoea index, hs-CRP, high-sensitive C-reactive protein; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; SaO2, oxygen saturation.
Discussion
This study provides several major insights into the short-term effects of ASV therapy on cardiac SNA in HF patients with CSA. First, positive correlations were found between the MIBG scintigraphic parameters and AHI score, apnoea index, central apnoea index, and hypopnoea index in HF patients. These results suggest that CSA, particularly abnormal breathing caused by HF, could be associated with elevated cardiac SNA. Secondly, a significant reduction in the WR and elevation in the H/M ratio were observed after 6 months of ASV therapy in HF patients with CSA, suggesting that the respiratory support provided by ASV therapy may be a therapeutic tool for decreasing SNA in HF patients with CSA. Thirdly, multiple linear regression analyses showed that the relative change in WR was a strong indicator of an improvement in the LVEF, suggesting that the reduced SNA caused by ASV therapy could contribute to improved cardiac contractile function.
Elevated SNA is closely associated with the occurrence of impaired cardiac contractile function, malignant arrhythmic events, and haematological abnormalities,18 resulting in a poor prognosis for HF patients.2 Hypocapnoea and fluctuating central chemoreflex sensitivity due to LV contractile dysfunction cause CSA, particularly abnormal breathing such as repeated CSA and hypopnoea.19 A reduced tidal volume due to an abnormal breathing pattern could cause reduced pulmonary vagal afferents, resulting in activated systemic SNA.20 Moreover, rapid, shallow breathing, which is frequently observed in HF patients,21 arousal responses during the hyperventilation following apnoea events, and hypoxic stress caused by CSA could also be an important contributor to elevated SNA.22 Consequently, the decreased cardiac function in HF patients might facilitate the appearance of CSA, which could be associated with enhanced cardiac SNA.23 It is likely that this vicious cycle is one of the mechanisms underlying the poor prognosis of HF patients. Reduced SNA could decrease systemic vascular resistance, resulting in the reduced afterload and higher stroke volume, in which inhibition of SNA could be an important therapeutic target for the improvement of HF patients.
It is well established that cardiac MIBG scintigraphy is a reliable diagnostic tool for evaluating cardiac SNA and the prognosis of HF patients.24 Previous studies of patients without cardiac disease showed that the WR was significantly higher while the H/M ratio was significantly lower in patients experiencing obstructive sleep apnoea (OSA) than in those without. One clinical study showed that the WR was significantly correlated with CSA in HF patients but not with OSA events, implying that HF patients with CSA experience greater norepinephrine spillover than those with OSA.25 These results suggest that abnormal breathing caused by HF is closely associated with cardiac SNA, and support our results showing that the severity of abnormal respiration is correlated with the WR and/or the H/M ratio acquired from MIBG scintigraphy.
Adaptive servo-ventilation therapy is an effective non-pharmacological therapeutic option for patients with HF.13,15 The ASV device maintains the minute ventilation according to a self-adjustment function without inhibiting a patient's breathing efforts, and it provides low-grade positive end-expiratory pressure, resulting in improved patient compliance and a better haemodynamic state.3 As shown in Figure 2, a decreased WR and increased H/M ratio were found in HF patients 6 months after the initiation of ASV therapy, indicating that ASV therapy can improve cardiac SNA. Although the underlying mechanisms for the improvement of cardiac SNA by ASV therapy have not been fully determined, a previous study showed that preventing abnormal breathing patterns through ASV therapy reduces muscle SNA immediately, whereby mechanical respiratory support enhances pulmonary vagal afferents and reduces SNA.9 A very recent study showed that several months of ASV therapy reduced muscle sympathetic nerve activity, together with the reduction of CSA events.26 The regulation of SNA by ASV therapy could be an important approach for the improvement of cardiac function. The other mechanism is that the haemodynamic support provided by ASV therapy may improve cardiac contractile function, resulting in reductions in abnormal breathing by enhancing cardiac SNA. A previous study indicated that ASV therapy converted rapid shallow breathing patterns to slow regular breathing patterns and prevented respiratory oscillations in HF patients.9 Respiratory control is an important contributor to the regulation of the autonomic nervous system in humans.8 Improving respiratory oscillations using ASV therapy could reduce cardiac SNA. Moreover, preventing hypoxic events with the use of ASV therapy could be an important underlying mechanism for improving cardiac SNA. Taken together, ASV therapy reduces cardiac SNA through these polymodal actions, and may lead to improved cardiac function.
The limitations of this study are as follows. First, the study population consisted of a small number of HF patients, so that the statistical analyses, including the multivariate analyses, might be inaccurate. Secondly, another therapeutic option such as nocturnal oxygen therapy should be compared with ASV to verify the results. Thirdly, this study could not be designed using a randomized protocol. Fourthly, these results were based on findings from Japan, and data from Europe and North America need to be considered. Fifthly, a pathophysiological approach using microneurography and muscle sympathetic nerve activity is needed to clarify the mechanism behind the reduction in SNA using ASV therapy. A larger number of randomized trials will be needed to resolve these limitations.
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Acknowledgements
The authors thank the participants for their cooperation and thank the staff of the Sleep Center at Akita University Hospital for their help.
Conflict of interest: none declared.




