Global left ventricular longitudinal strain is closely associated with increased neurohormonal activation after acute myocardial infarction in patients with both reduced and preserved ejection fraction: a two-dimensional speckle tracking study
Abstract
Aims
N-terminal pro brain natriuretic peptide (NT-proBNP) is released in response to increased myocardial wall stress and is associated with adverse outcome in acute myocardial infarction. However, little is known about the relationship between longitudinal deformation indices and NT-proBNP.
Methods and results
We prospectively included 611 patients with acute myocardial infarction admitted to a tertiary centre and performed echocardiography within 48 h of admission. Global longitudinal myocardial function was assessed by two-dimensional speckle tracking simultaneously with measurement of plasma NT-proBNP. A significant linear relationship between NT-proBNP and global longitudinal strain (GLS) was found (P < 0.0001, r = 0.62). Weaker correlation was found between NT-proBNP and left ventricular ejection fraction (LVEF; P < 0.0001, r = – 0.44). GLS emerged on multivariable analysis including age, sex, estimated glomerular filtration rate, Killip class ≥2, diabetes, hypertension, presence of ST segment elevation, anterior infarction, troponin level, left atrial volume index, mitral valve deceleration time, and E/e' as the strongest predictor of log(NT-proBNP) (P < 0.0001). In patients with preserved systolic function (LVEF >45%), GLS remained strongly correlated with NT-proBNP (P < 0.0001, r = 0.50). The C-statistic associated with prediction of upper vs. lower quartiles of NT-proBNP was significantly higher for GLS compared with LVEF (0.76 vs. 0.56; P < 0.0001).
Conclusion
Left ventricular longitudinal function assessed by GLS exhibits a stronger association with NT-proBNP levels in acute myocardial infarction compared with LVEF. In patients with apparently preserved systolic function, GLS is superior to LVEF in identifying increased neurohormonal activation.
Introduction
Risk assessment of patients presenting with acute myocardial infarction (MI) is of paramount importance in order optimally to select patients for therapies that may improve the outcome. Currently risk stratification is based on electrocardiogram (ECG) changes, biochemical markers of cardiomyocyte necrosis, clinical history, and echocardiographic evidence of reduced left ventricular (LV) ejection fraction (LVEF).1,2 The N-terminal fragment of pro brain natriuretic peptide (NT-proBNP) is enzymatically cleaved from the prohormone proBNP and, according to our current understanding, secreted by cardiomyocytes in response to increased wall tension.3 Elevated levels of NT-proBNP have been shown to reflect adverse prognosis across the spectrum of acute coronary syndromes (ACS);4–6 however, routine measurements of NT-proBNP are not incorporated in guidelines for ACS. The levels of NT-proBNP are inversely related to LVEF in a variety of cardiac pathologies, and this relationship is even stronger in patients with clinical heart failure.7 NT-proBNP correlates with the severity of diastolic dysfunction in systolic heart failure;8 furthermore, the severity of diastolic dysfunction correlates well with levels of NT-proBNP in patients with heart failure and preserved ejection fraction (HF-PEF), and contains independent prognostic information in this population.9,10 Recently NT-proBNP measured at the time of hospitalization for MI has been shown to correlate with infarct size measured by magnetic resonance imaging (MRI) at follow-up.11,12 Finally elevated levels of NT-proBNP are associated with adverse outcome in unselected patients presenting in the Emergency Department.13
Longitudinal strain can quantify the systolic function of the longitudinal fibres and allows for the evaluation of regional and global (GLS) deformation properties of the myocardium. The longitudinal fibres in the subendocardial layer are more sensitive to ischaemia and wall stress, and can exhibit abnormal contraction patterns in the setting of apparently normal LVEF. Deformation imaging using GLS thus allows for a more sensitive method to identify subclinical LV systolic dysfunction.14,15
Furthermore, data suggest that GLS is able to reflect more accurately the final infarct size after MI compared with LVEF.16 However, limited data exist on the relationship between deformation parameters measured by echocardiography and neurohormonal activation. To test the hypothesis that neurohormonal activation is more closely related to longitudinal deformation than traditional markers of LV systolic function, NT-proBNP was determined and comprehensive echocardiography was prospectively performed in a large cohort of patients with MI.
Methods
Study design and patient population
We conducted a prospective study of patients referred to our centre for invasive coronary angiography (CAG) due to either ST segment elevation MI (STEMI) or non-ST segment elevation MI (NSTEMI) from September 2009 to October 2010. All patients provided written informed consent prior to transthoracic echocardiographic examination and blood sampling. Exclusion criteria were age <18 years, non-cardiac disease with a life expectancy <1 year, or inability to provide written informed consent.
The patients were prospectively classified according to ECG changes based on the presence of ST segment elevation.17 The final MI diagnosis was based on elevated troponin T in all patients. Based on hospital records obtained at admission, diabetes mellitus, hypertension, a history of ischaemic heart disease, prior MI, and pre-existing congestive heart failure was registered. Clinical events from the arrival of emergency services and during hospitalization were recorded including the occurrence of cardiac arrest, sustained ventricular arrhythmias, and supraventricular arrhythmias. Findings in relation to CAG including culprit lesion, number of diseased vessels, and type of revascularization [percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), or no intervention] were registered. The study was approved by the Regional Scientific Ethics Committee (reference number H-D-2009-063).
Blood sampling procedures and assay of N-terminal pro brain natriuretic peptide
Peripheral samples of plasma were obtained after written informed consent within 24 h of echocardiography. Analysis of NT-proBNP was performed on the commercially available Modular Analytics E170 NT-proBNP immunoassay (Roche Diagnostics, Mannheim, Germany) immediately after blood sampling. In addition, routine biochemical work-up was performed including creatinine, haemoglobin, LDL cholesterol, glycated haemoglobin (HbA1C), sodium, potassium, and peak troponin T during the hospital stay. Estimated glomerular filtration rate (eGFR) was measured from the four-variable Modification of Diet in Renal Disease (MDRD) formula.
Echocardiography and two-dimensional speckle tracking
Echocardiography was performed within 48 h of admission to our institution. Patients with STEMI were examined after the invasive procedure and patients with NSTEMI just prior to CAG. Echocardiographic cine loops were obtained by recording three consecutive heart cycles with 120 ms before and after the first and last QRS complex, respectively. All examinations were performed on a Vivid e9 (General Electric, Horten, Norway). Images were obtained at a frame rate of 60–75/s and digitally transferred to a remote workstation for offline analysis (Echopac BT 11.1.0, General Electric, Horten, Norway). All analyses were performed by a single experienced operator blinded to clinical, biochemical, and CAG information.
Two-dimensional parasternal images were used to determine LV dimensions and wall thickness. Left atrial volume index (LAVi) was determined from the biplane area length method, and LV volumes were determined using the biplane Simpson model. LV mass was calculated from the LV linear dimensions in the parasternal view. Volumetric and dimensional measurements were indexed to body surface area when appropriate. All volumetric analyses were performed in accordance with the EAE/ASE (European Association of Echocardiography/American Society of Echocardiography).18
Doppler recordings of mitral inflow were performed by placing a 2.5 mm sample volume at the tip of the mitral valve (MV) leaflets and recording the pulsed wave Doppler signal. Peak velocity of early (E) and atrial (A) diastolic filling and MV deceleration time (DT) were measured and the E/A ratio calculated. Continuous wave Doppler recording of the LV outflow tract were recorded, and aortic valve opening and closure times were measured. Pulsed wave (PW) tissue Doppler imaging (TDI) recordings were performed at the lateral and medial mitral annulus using a 2.5 mm sample volume with measurements of myocardial peak early (e') and late (a') diastolic velocities as well as peak systolic velocity (s'). The E/e' ratio was calculated from the average of lateral and medial e' values. All TDI recordings were performed with frame rates >130/s.
Two-dimensional speckle tracking was performed using a semi-automatic algorithm (Automated Function Imaging, GE). Briefly, manual positioning of three points (two annular and one apical) was performed in each of the three apical projections, enabling the software to track the myocardium semi-automatically throughout the heart cycle. Each ventricular wall was subsequently divided into three segments for a total of 17 segments covering the entire myocardium. Careful manual inspection of tracking was performed, and in the case of unsatisfactory tracking the segment would be excluded from the analysis. Longitudinal strain curves were generated for each segment and the maximum value was calculated. The GLS was then calculated as the average of all 17 segments (Figure 1).
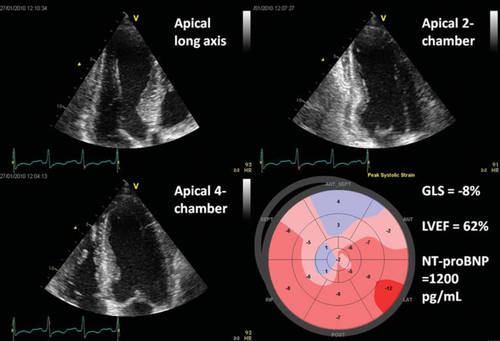
Statistical analysis
Data analysis was performed using SAS Software 9.2 (Cary, NC, USA). All data are reported as mean ± standard deviation (SD) or median when appropriate. Regression analysis was performed in a general linear model allowing for inclusion of continuous and categorical variables alike. NT-proBNP was logarithmically transformed (log10) in order to stabilize the variance in accordance with the assumptions of multiple regression analysis. A P-value < 0.05 was considered significant, and the explained variation of the general linear model was derived from the global R2 value. All variables with suspected clinical relevance were entered and the parsimonious model obtained by subsequent variable elimination in a backward stepwise manner with P < 0.1 as a retention criterion. We adjusted for age, sex, diabetes, hypertension, eGFR, Killip class ≥2, infarct type (STEMI/NSTEMI), troponin T level, left anterior descending (LAD) involvement, multivessel disease, LAVi, LV mass, E/e', MV deceleration time, and GLS. Interaction terms were included in the multiple regression analysis to examine the potential modulating effect of clinically relevant covariates on the explanatory value of GLS and formally rejected if found non-significant (P > 0.05). Values of NT-proBNP in the upper quartile were compared with those in the lower quartile and separate logistic regression models were constructed for GLS and LVEF as the single predictor variable. Comparison of each method's predictive capability was performed by comparing the C-statistic derived from the area under the receiver operating characteristic (ROC) curves using the generalized U-statistic as proposed by DeLong et al.19 The validity of the difference was tested by regenerating the data set 1000 times by bootstrapping using random resampling by replacement. The bootstrapped data sets were used to regenerate the two logistic regression models and the resampled C-statistics were subsequently compared with a paired t-test. Separate identical analyses were performed for patients with preserved LVEF. Finally, 20 patients were randomly selected to test the intra- and interobserver variability for automated function imaging GLS measurements by Bland–Altman analysis.
Results
The total study population consisted of 611 patients. Twenty two-patients were excluded from the analysis due to atrial fibrillation (n = 20) and ventricular paced rhythm (n = 2). Forty-one patients were excluded due to poor image quality causing three or more myocardial segments to be incorrectly tracked by the speckle tracking algorithm. Thus 548 patients were included in the analyses (mean age 63.1 ± 12 years, 71.5% male) of which 370 had STEMI (67.5%) and 178 had NSTEMI (32.5%). In STEMI patients, the median symptom to balloon time was 197 min (interquartile range 147–310). The level of NT-proBNP ranged from 5.0 to 8180 pmol/L, with a median of 115.5 pmol/L (interquartile range 48.3–246.0). The subgroup of patients with LVEF >45% comprised 387 individuals (mean age 62.3 ± 11.7 years, 71% male) of which 254 had STEMI (65.6%) and 133 had NSTEMI (34.4%). NT-proBNP ranged from 5.0 to 3525 pmol/L, with a median of 88.7 pmol/L (interquartile range 36.1–170.0). Thirty-three patients died within 1 year after entering the study for a 1-year mortality of 5.6%, precluding the determination of the relative prognostic importance of GLS compared with NT-proBNP. Baseline demographics of the total study population and patients with preserved LVEF are presented in Table 1.
| Total (n = 548) | LVEF >45% (n = 387) | |
|---|---|---|
| Age, years | 63.2 (11.7) | 62.3 (11.7) |
| Female, n (%) | 156 (28.5%) | 112 (28.9%) |
| BMI, kg/m2 | 27.4 (13.9) | 27.6 (16.3) |
| History of hypertension, n (%) | 254 (46%) | 178 (46.0%) |
| History of smoking, n (%) | 367 (66.7%) | 262 (67.7%) |
| Diabetes, n (%) | 75 (13.7%) | 43 (11.1%) |
| History of IHD, n (%) | 95 (17%) | 60 (15.5%) |
| Known HF, n (%) | 33 (6.0%) | 16 (4.2%) |
| Known COPD, n(%) | 37 (6.8%) | 24 (6.3%) |
| eGFR, mL/min | 90.6 (28.6) | 92.6 (28.1) |
| Peak troponin T, pg/L | 3.90 (5.0) | 3.22 (1.7) |
| Killip class | ||
| 1 | 459 (83.8%) | 353 (91.1%) |
| 2 | 52 (9.5%) | 25 (6.5%) |
| 3 | 27 (4.9%) | 6 (1.6%) |
| 4 | 10 (1.8%) | 3 (0.8%) |
| Type of infarction | ||
| STEMI, n (%) | 370 (67.5%) | 254 (65.6%) |
| NSTEMI, n (%) | 178 (32.5%) | 133 (34.4%) |
| Angiographic findings | ||
| LAD involvement, n (%) | 209 (38.1%) | 129 (33.3%) |
| Multivessel disease, n (%) | 214 (39.1%) | 130 (33.4%) |
| Treatment decision | ||
| PCI, n (%) | 103 (18.8%) | 80 (20.7%) |
| Primary PCI, n (%) | 333 (60.8%) | 233 (60.2%) |
| No invasive treatment, n (%) | 74 (13.5%) | 56 (14.5%) |
| CABG, n (%) | 38 (6.9%) | 18 (4.6%) |
| NT-proBNP, median (range) | 111.5 (5–8175) | 88.7 (5–3530) |
- a BMI, body mass index; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; IHD, ischaemic heart disease; LAD, left anterior descending; LVEF, left ventricular ejection fraction; NSTEMI, non-ST segement elevation myocardial infarction; NT-proBNP, N-terminal pro brain natriuretic peptide; PCI, percutaneous coronary intervention; STEMI, ST segment elevation myocardial infarction.
The overall linear relationships between log(NT-proBNP) and GLS and LVEF are shown in Figure 2. Correlation between GLS and log(NT-proBNP) was significant (P < 0.0001, r = 0.62). The observed correlation of log(NT-proBNP) with LVEF was significant but explained a smaller magnitude of the variance (P < 0.0001, r = –0.44). Weaker correlation was found between TDI PW systolic annular velocity (s') and log(NT-proBNP) (P < 0.001 r = –0.27). In patients with STEMI and NSTEMI analysed separately, log(NT-proBNP) exhibited a stronger overall correlation with GLS (STEMI, P < 0.0001, r = 0.61; NSTEMI, P < 0.0001, r = 0.57) compared with LVEF (STEMI, P < 0.0001, r = –0.45; NSTEMI, P < 0.0001, r = –0.42). In patients with preserved LVEF, GLS remained strongly correlated with log(NT-proBNP) (P < 0.0001, r = 0.50) (Figure 3), whereas the correlation between LVEF and log(NT-proBNP) weakened considerably (P < 0.0001, r = –0.21). When analysing STEMI and NSTEMI patients separately, GLS (STEMI, P < 0.0001, r = 0.46; NSTEMI, P < 0.0001, r = 0.45) remained strongly correlated with log(NT-proBNP), whereas LVEF [STEMI, P < 0.001, r = –0.23; NSTEMI, P = non-significant (NS), r = –0.12] weakened significantly in patients with STEMI and lost significance in patients with NSTEMI. The correlation between GLS and LVEF was significant for the total study population (P < 0.0001, r = –0.66) and weaker in patients with preserved LVEF (P < 0.0001, r = –0.44).
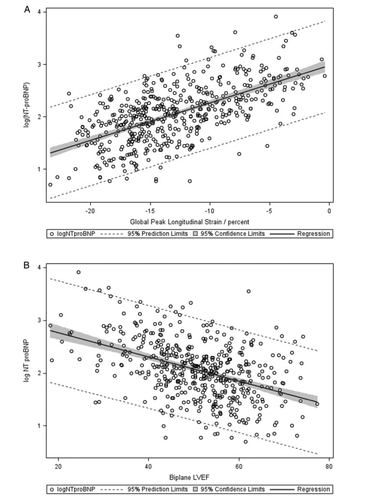
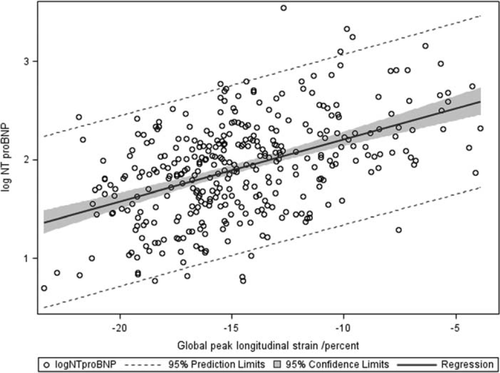
In multiple regression analysis GLS emerged as an independent predictor of log(NT-proBNP), with no significant explanatory value of diabetes, hypertension, LAD involvement, multivessel disease, LAVi, or LV mass. Interaction terms were analysed for interdependence between GLS and age, sex, Killip class ≥2, diabetes, and LV mass, and were all found to be non-significant. Furthermore, the interaction between type of infarction and GLS was found to be non-significant both overall and in patients with preserved LVEF. The parsimonious model included age, sex, eGFR, troponin T, MV deceleration time, E/e', and GLS (β = 0.05, P < 0.0001; overall R2 = 0.56). Assessing the parameter estimate of the β value for GLS and back-transforming NT-proBNP, we estimated that reducing GLS by one absolute percentage point would be associated with an increase of NT-proBNP of 12.4% [confidence interval (CI) 10.3–14.5%] when all other covariates in the model were held constant. In patients with preserved LVEF, GLS continued to be independently associated with log(NT-proBNP) in a parsimonious multiple regression model including age, sex, Killip class ≥2, and troponin T (β = 0.04, P < 0.0001, overall R2 = 0.46). The results of the multiple regression analyses are given in Table 2 along with β-values and associated CIs.
| Parameter | Total (n = 548) | LVEF >45% (n = 387) | ||
|---|---|---|---|---|
| β (P-value) | 95% CI | β (P-value) | 95% CI | |
| Age | 0.014 (<0.0001) | 0.010–0.017 | 0.014 (<0.0001) | 0.011–0.017 |
| Female sex | 0.20 (<0.0001) | 0.11–0.28 | 0.192 (<0.0001) | 0.104–0.280 |
| Killip class ≥2 | ns | 0.217 (<0.005) | 0.071–0.362 | |
| eGFR (per 10 mL decrease) | 0.01 (<0.05) | 0.01–0.03 | NS | |
| Troponin T | 0.023 (<0.0001) | 0.016–0.030 | 0.037 (<0.0001) | 0.026–0.048 |
| MV deceleration time (per 10 ms decrease) | 0.01 (<0.01) | 0.01–0.02 | NS | |
| E/e' ratio (per 5 unit increase) | 0.04 (<0.05) | 0.002–0.080 | NS | |
| GLS | 0.050 (0.0001) | 0.043–0.059 | 0.040 (<0.0001) | 0.029–0.052 |
- a Parameter estimates for β-values are per 1 unit increase of the covariate unless otherwise stated.
- b eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; MV, mitral valve.
Univariate logistic regression analysis was performed separately for GLS and LVEF, with a NT-proBNP dichotomized into lower and upper quartile values. In the overall study population, GLS [odds ratio (OR) 1.4, P < 0.0001; CI 1.30–1.51] and LVEF (OR 0.92, P < 0.0001; CI 0.90–0.94) were both significantly associated with dichotomized NT-proBNP categories; however, the C-statistics for GLS weresignificantly higher than for LVEF (0.86 vs. 0.75, P < 0.0001). ROC curves for GLS and LVEF are shown in Figure 4A. In patients with preserved systolic function, both GLS (OR 1.30, P < 0.0001; CI 1.19–1.42) and LVEF (OR 0.95, P < 0.05; CI 0.91–0.99) continued to be significantly associated with dichotomized NT-proBNP levels; however, the explanatory value of LVEF was diminished. Furthermore, the difference in the C-statistics associated with dichotomized NT-proBNP categories between GLS and LVEF was increased (0.76 vs. 0.61, P < 0.0001) (Figure 4B). To assess the internal validity of the differences of the C-statistics for GLS and LVEF, we performed bootstrapping as mentioned previously. In the overall comparison, the C-statistic of GLS (0.858; CI 0.856–0.860) was significantly higher when compared with LVEF (0.749; CI 0.747–0.751), P < 0.0001. In the group with preserved LVEF, the C-statistic of GLS (0.759; CI 0.756–0.761) outperformed that of LVEF (0.617; CI 0.615–0.620). Bland–Altman analysis demonstrated a good intra- and interobserver agreement with a small non-significant bias for GLS. Mean difference ± 2 SD for GLS was –0.7 ± 2.5% and –0.5 ± 1.3% for inter- and intraobserver agreement, respectively.
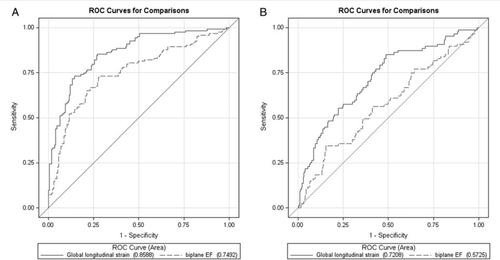
Discussion
The main finding of this study is that assessment of longitudinal function by GLS has a significantly stronger relationship to the level of neurohormonal activation than LVEF in a large cohort of patients in the acute phase of MI. Furthermore, the diagnostic accuracy as expressed by the C-statistic of GLS outperformed that of LVEF. These findings were robust when resampling by bootstrapping was performed. Importantly, in a large subgroup of patients with preserved LVEF and thus apparently normal systolic function, GLS continued to demonstrate a clear linear relationship with log(NT-proBNP) compared with the markedly weakened association with LVEF. Neither correlations nor interaction analysis indicated a different relationship between GLS and NT-proBNP according to the type of infarction (STEMI/NSTEMI). These findings suggest that in MI, GLS may be a more accurate echocardiographic marker of systolic impairment than LVEF.
The observed relationship between GLS and NT-proBNP could indeed reflect a pathophysiological connection between longitudinal fibre dysfunction and secretion of natriuretic peptides. This would be inadequately described by LVEF which to a greater extent quantifies overall volume displacement without detailed information on myocardial function. A previous study found a negative correlation between longitudinal annular systolic velocities (s') measured by TDI in relation to levels of NT-proBNP20 in a large community-based cohort. These findings were confirmed in a smaller cohort of patients with suspected heart failure, where longitudinal velocity predicted BNP and subsequently a diagnosis of heart failure.21 Thus depressed longitudinal function as expressed by annular velocity is related to NT-proBNP levels. However, caution is warranted in extrapolating the findings in the setting of chronic heart failure to the acute phase of MI, where remodelling and fibrosis are less likely to contribute to neurohormonal activation. We found significantly stronger correlations between GLS and NT-proBNP compared with that between s' and NT-proBNP. We speculate that these findings reflect that s' to a lesser extent than GLS reflects the heterogeneous contraction associated with MI and that s' is more susceptible to tethering. Furthermore, a recent study demonstrated that deformation indices rather than myocardial velocity identified systolic dysfunction in HF-PEF patients.22
Biplane LVEF calculation utilizing the Simpson method is dependent on visual delineation of the endocardial border, which can be a challenge in patients with poor acoustic conditions. This limitation could contribute to the superiority of GLS over LVEF; however, the same challenges are present when performing two-dimensional speckle tracking. Time consumption for GLS measurements has previously been reported and it exceeds that of LVEF calculation in the range of 20 s.14
A major determinant of NT-proBNP in patients with chronic heart failure is believed to be wall stress (WS) which is determined by LV diastolic filling pressure, wall thickness, and LV diastolic diameter. Thus for increasing filling pressures given a constant wall thickness in the absence of compensatory concentric hypertrophy, the WS will increase. We found a small but significant effect of the E/e' ratio and MV deceleration time in multivariable modelling in the overall analysis; however, in patients with preserved LVEF these effects were insignificant. An elevated E/e' ratio and shortened MV deceleration time contain prognostic information in patients with MI and reflect increasingly restrictive filling, elevated filling pressure, and increased WS.23
The relationship between WS and BNP was explored by Iwanaga et al. in a cohort of 160 heart failure patients where a strong positive correlation was observed, and this was consistent when patients were divided according to systolic and diastolic heart failure.24 The relationship between longitudinal function and WS has not been explored in detail with invasive haemodynamic monitoring in humans; however, an animal study by Donal et al. clearly demonstrated that longitudinal function was more susceptible to increased WS whereas intact circumferential function served to preserve radial fractional shortening of the cavity diameter.25
The complex anatomy of the myocardium consists of subendocardial longitudinally oriented fibres with an oblique direction, whereas mid-wall fibres are circumferentially oriented. This results in a non-uniform distribution of WS, with decreasing values from endo- to epicardium. The subendocardial longitudinal fibres have a smaller curvature than the mid-wall circumferential fibres, thus the WS according to the law of Laplace governing a three-dimensional shell will be higher on the longitudinal fibres.26,27
A potential explanation of the closer association between GLS and NT-proBNP compared with LVEF could also be the insensitivity of these measures in reflecting the function of areas in the peri-infarct zone as well as the non-ischaemic surrounding myocardium. Rapid induction of BNP gene expression occurs in these areas, which has been demonstrated in animal experiments,28 and BNP elevation in the setting of even transient ischaemia during PCI has been demonstrated in stable patients.29 These findings along with the fact that subendocardial longitudinal fibres are especially sensitive to ischaemia21 and experimental demonstration of up-regulated BNP mRNA in the endocardial region30 lend explanatory support to the findings in our study.
The NT-proBNP levels during initial hospitalization in patients with STEMI have been compared with infarct size assessed by late gadolinium enhancement MRI, and positive correlations are reported.11,12 Furthermore, initial NT-proBNP above the mean in the study population was associated with low probability of functional recovery as assessed by regional wall thickening at follow-up. Both studies were small in size, included only STEMI patients, and excluded patients with prior ischaemic heart disease as well as patients with in-hospital symptoms of decompensated heart failure (Killip class ≥2).
Finally, GLS has been shown to be able to discriminate between large, medium, and small infarct size16 with greater accuracy than LVEF, thus GLS as a surrogate for final infarct size could explain our findings in relation to NT-proBNP.
Study limitations
A limitation in this study is that NT-proBNP measurements were not conducted at one standardized time point in all patients. Even though blood sampling was performed within 24 h of echocardiography we cannot exclude the possibility that this could influence the results. However, several large-scale studies have provided solid evidence for the prognostic utility of BNP and NT-proBNP in the setting of STEMI as well as NSTEMI on top of and in addition to existing known risk factors for adverse events after MI regardless of the chosen time point of blood sampling.31 We define preserved systolic function as LVEF >45% and thus a priori weaken the correlation between LVEF and NT-proBNP. However, all therapies targeting remodelling after MI are currently only recommended for patients with LVEF <45%, and increasingly NT-proBNP is advocated as an additional risk marker across all levels of LV dysfunction. We therefore find that stratifying according to LVEF is justified.
Implications
The findings in the present study demonstrate that GLS reflects neurohormonal activation in the setting of MI and that in patients with preserved LVEF increased levels of NT-proBNP are closely related to longitudinal functional impairment. The automated assessment of GLS from three standard apical projections reveals systolic impairment reflected by increased neurohormonal activation and not captured by the calculation of LVEF. These findings warrant further studies to clarify whether measurement of GLS during the acute phase of MI modifies the prognostic value of NT-proBNP and, importantly whether the combination of GLS and NT-proBNP can identify high risk individuals with preserved LVEF. Finally, elevated NT-proBNP levels during hospitalization for MI can be echocardiographically validated by assessment of longitudinal deformation.
Conclusion
Plasma levels of NT-proBNP in the acute phase of MI are more accurately reflected by longitudinal myocardial deformation assessed by two-dimensional speckle tracking-derived GLS compared with LVEF. This relationship is even more pronounced when LVEF is preserved. Thus, a more accurate measure of systolic function is obtained by the assessment of GLS. In patients with preserved LVEF and elevated levels of NT-proBNP in the acute phase of MI, particular attention should be paid to longitudinal dysfunction.
Conflict of interest: none declared.




