Dipeptidyl peptidase IV inhibition improves cardiorenal function in overpacing-induced heart failure
Abstract
Aims
Recent studies indicate that brain natriuretic peptide (BNP1–32) may be truncated into BNP3–32 by dipeptidyl peptidase IV (DPP4) and that BNP3–32 has reduced biological activities compared with BNP1–32. We investigated if DPP4 contributes to the cardiorenal alterations and to the attenuated response to BNP seen in heart failure.
Methods and results
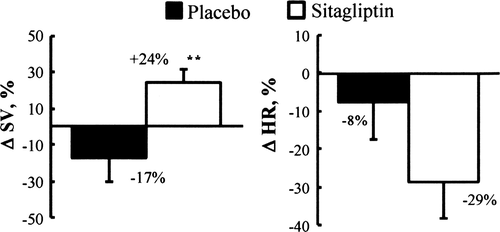
Haemodynamic and renal assessment was performed in 12 pigs at baseline, 4 weeks after pacing-induced heart failure, and during BNP infusion. They were randomized to either placebo or treatment with a DPP4 inhibitor, sitagliptin. After 4 weeks of pacing, heart rate was reduced compared with baseline in the sitagliptin group (60 ± 2 vs. 95 ± 16 b.p.m., P < 0.01), and an increase in stroke volume was observed in the sitagliptin group compared with placebo (+24 ± 6% vs. –17 ± 7%, P < 0.01). Glomerular filtration rate declined at week 4 compared with baseline in the placebo group (1.3 ± 0.4 vs. 2.3 ± 0.3 mL/kg/min, P < 0.01) but remained preserved in the sitagliptin group [1.8 ± 0.2 vs. 2.0 ± 0.3 mL/kg/min, P = NS (non-significant)]. In the sitagliptin group, BNP infusion improved end-systolic elastance (68 ± 5 vs. 31 ± 4 mmHg/kg/mL, P < 0.05), ventricular–arterial coupling, and mechanical efficiency. Compared with controls (n = 6), myocardial gene expression of BNP, interleukin-6, Na+–Ca2+ exchanger, and calmodulin was up-regulated in the placebo group, but not in the sitagliptin group.
Conclusion
In pacing-induced heart failure, DPP4 inhibition preserves the glomerular filtration rate, modulates stroke volume and heart rate, and potentiates the positive inotropic effect of exogenous BNP at no energy expense.
Introduction
Brain natriuretic peptide (BNP1−32) is produced by ventricular cardiac myocytes in response to increased wall stress. BNP1−32 exerts various cardiorenal effects such as increased natriuresis, vasodilation, and improved haemodynamics. The up-regulation of BNP in response to overload is protective and considered to be a potential therapeutic tool in the treatment of heart failure (HF).1 However in HF patients, the beneficial effects of BNP are not always apparent.2 The reason is multifactorial but may be explained in part by the presence of several BNP molecular entities in blood, each with different biological effects.3,4 Obviously, knowing how to modulate the formation of truncated BNP may have an impact on the therapeutic approach to patients with HF.
In vitro, BNP1–32 is cleaved into BNP3–32 by the ubiquitous enzyme dipeptidyl peptidase IV (DPP IV, DPP4).5 BNP3–32 has reduced biological activities compared with BNP1–32.6 This observation seems even more clinically relevant when we consider that BNP3–32 is increased in patients with chronic HF.7,8 Moreover, circulating BNP1–32 was found to be absent in a small group of patients with severe HF using a highly sensitive and specific mass spectrometry technique.9 Very recently, human proBNP was shown to be processed in rat circulation and led to the production of mature BNP1–32 along with proteolytically truncated BNP forms such as BNP3–32.10
Therefore, we sought to investigate if DPP4 might contribute to the cardiorenal alterations and to the attenuated response to BNP seen in HF. To test this, a model of pacing-induced dilated cardiomyopathy in pigs was used, with each animal being exposed to chronic treatment with sitagliptin, an oral DPP4 inhibitor, or placebo. In addition, we evaluated directly whether the intravenous infusion of porcine BNP acutely modulates left ventricular (LV) haemodynamics in sitgaliptin-treated animals with HF.
Methods
Study design
The design of the placebo-controlled, prospective, randomized, parallel, and double-blinded study is summarized in Figure 1. A tachycardiomyopathy was induced in 12 female hybrid pigs (Landrace × Large White) weighing 23.9 ± 1.5 kg by ventricular overpacing at 190 b.p.m. over a 4 week period. Sitagliptin (30 mg/kg) (Januvia 100 mg, MSD, Brussels, Belgium) was orally administrated once a day, in 6 of 12 animals from the end of week 1 until the end of the protocol, the last dose being administered on the morning of the final investigation.
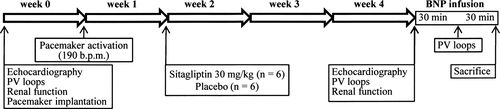
Cardiac and renal assessments were performed in sinus rhythm at baseline and at the end of the pacing period, under general anaesthesia as described in the Supplementary material online, Methods. In HF pigs, cardiovascular investigation was realized before BNP administration but also repeated 30 min after starting intravenous infusion of porcine BNP (H2952, Bachem, Budendorf, Switzerland, 2 μg/kg in bolus then 0.02 μg/kg/min). After sacrifice, tissue samples were procured for histological and molecular analyses. Samples of the LV free wall from six control pigs were used for comparisons.
Sitagliptin dosage
DPP4 activity was measured as described in Supplementary material online, Methods.
Echocardiography
Echocardiography (Vivid 5, GE, Brussels, Belgium) was carried out with a 5 MHz electronic probe as described in Supplementary material online, Methods.
Invasive assessment of LV haemodynamics
Instantaneous LV pressure–volume (PV) loops were generated in anaesthetized closed chest pigs with a combined dual sensor PV 6 F pigtail catheter (Millar Instruments Inc., Houston, TX, USA) connected to a Leycom Sigma-5 signal conditioner processor (Cardiodynamics, Leiden, The Netherlands) as described in Supplementary material online, Methods.
Renal function
The glomerular filtration rate (GFR) and renal plasma flow (RPF) were measured by the exogenous creatinine and PAH (p-aminohippurate) clearances, respectively, as described in Supplementary material online, Methods.
Autopsy and histology
Assessment of myocardial inflammation, fibrosis, capillary density, and cardiac myocyte hypertrophy was conducted as described in Supplementary material online, Methods.
Quantitative real-time polymerase chain reaction (QRT-PCR) and enzyme-linked immunosorbent assay (ELISA)
Primers were designed to recognize pig cDNA sequences of sarcoplasmic reticulum Ca-ATPase (SERCA), phospholamban (PLB), sarcolemmal Na+–Ca2+ exchanger (NCX), calmodulin (CaM), adrenergic β1 receptor (β1-AR), BNP, tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), monocyte chemotactic protein 1 (MCP1), stromal cell-derived factor-1 (SDF-1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). QRT-PCR was performed as described in Supplementary material online, Methods. Myocardial SDF-1 protein levels were measured by ELISA as described in Supplementary material online, Methods.
Statistical analysis
When results of the Shapiro–Wilk test indicated that the variables were normally distributed, echocardiographic, haemodynamic, and renal variables were tested by a two-factor analysis of variance (ANOVA) for repeated measures, while post-mortem parameters (histology, QRT-PCR, and ELISA) were tested by a one-factor ANOVA, followed by Scheffe post-hoc tests when overall significance was detected. When the Shapiro–Wilk test failed, differences between groups were tested by using a Mann–Whitney test.
Results
Effects of chronic dipeptidyl peptidase IV inhibition on dipeptidyl peptidase IV activity and glycaemia
In sitagliptin-treated animals, plasma DPP4 activity was depressed at the end of the pacing period compared with baseline. The measured value of 4.9 ± 1.1 U/L after sitagliptin therapy vs. 20.7 ± 1.6 U/L at baseline (P < 0.01) overestimates DPP4 activity after treatment because of the dilution and re-equilibration during the DPP4 assay in vitro. The estimated mean in vivo inhibition of plasma DPP4 activity in the sitagliptin-treated group was 95.2 ± 0.6%. Glycaemia was lower in the sitagliptin-treated animals (88.4 ± 3.8 mg/dL) than in the placebo group (102.4 ± 6.9 mg/dL, P < 0.05).
Haemodynamic and renal effects of chronic dipeptidyl peptidase IV inhibition
At baseline, echocardiographic, haemodynamic, and renal functional indices were similar between the placebo- and sitagliptin-treated animals. In both groups, 4 weeks of pacing resulted in an increase in LV end-diastolic and end-systolic volumes, the E point to septal separation, and the left atrial diameter/aortic diameter ratio in parallel with a reduction in LV ejection fraction. Nevertheless, the left atrial diameter/aortic diameter ratio was higher in the placebo vs. the sitagliptin-treated animals (Supplementary material online, Table S1).
Following invasive haemodynamic measurements, heart rate (HR) decreased significantly in the sitagliptin-treated group at 4 weeks vs. baseline. Stroke volume (SV) increased in the sitagliptin group despite the 4 weeks of pacing, while it declined after the same period of pacing in the placebo group (Table 1 and Figure 2).
| Baseline | End | ||
|---|---|---|---|
| SV (mL/kg) | Placebo | 1.69 ± 0.13 | 1.43 ± 0.28 |
| Sitagliptin | 1.42 ± 0.11 | 1.72 ± 0.13 | |
| HR (b.p.m.) | Placebo | 89 ± 11 | 81 ± 11 |
| Sitagliptin | 95 ± 16 | 60 ± 2** | |
| MAP (mmHg) | Placebo | 77 ± 3 | 78 ± 3 |
| Sitagliptin | 81 ± 3 | 77 ± 10 | |
| PAPm (mmHg) | Placebo | 17 ± 1 | 28 ± 4** |
| Sitagliptin | 16 ± 1 | 26 ± 3** | |
| PCWP (mmHg) | Placebo | 12 ± 1 | 17 ± 2* |
| Sitagliptin | 11 ± 1 | 15 ± 1 | |
| RAP (mmHg) | Placebo | 7 ± 1 | 10 ± 1 |
| Sitagliptin | 7 ± 1 | 11 ± 2 | |
| dP/dtmax (mmHg/s) | Placebo | 2002 ± 173 | 1226 ± 127** |
| Sitagliptin | 1790 ± 87 | 1430 ± 175 | |
| Ees (mmHg/kg/mL) | Placebo | 58 ± 12 | 20 ± 3** |
| Sitagliptin | 78 ± 14 | 31 ± 4** | |
| Ea (mmHg/kg/mL) | Placebo | 61 ± 7 | 77 ± 17 |
| Sitagliptin | 69 ± 9 | 64 ± 12 | |
| Ees/Ea | Placebo | 1.0 ± 0.2 | 0.25 ± 0.03** |
| Sitagliptin | 1.2 ± 0.3 | 0.50 ± 0.04** | |
| SW/PVA | Placebo | 0.22 ± 0.04 | 0.09 ± 0.01* |
| Sitagliptin | 0.27 ± 0.03 | 0.16 ± 0.02* | |
| GFR (mL/kg/min) | Placebo | 2.3 ± 0.3 | 1.3 ± 0.4** |
| Sitagliptin | 2.0 ± 0.1 | 1.8 ± 0.2 | |
| RPF (mL/kg/min) | Placebo | 4.3 ± 0.7 | 2.5 ± 0.8*** |
| Sitagliptin | 5.1 ± 0.6 | 4.0 ± 0.5* |
- a Data are presented as mean ± SEM
- * P < 0.05 compared with baseline
- ** P < 0.01 compared with baseline
- *** P < 0.001 compared with baseline. Placebo, n = 6 and sitagliptin, n = 6.
- e dP/dtmax, maximal rate of rise in ventricular pressure; Ea, effective arterial elastance; Ees, end-systolic elastance; GFR, glomerular filtration rate; HR, heart rate; MAP, mean systemic arterial pressure; PAPm, mean pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; PVA, pressure–volume area; RAP, right atrial pressure; RPF, renal plasma flow; SV, stroke volume; SW, stroke work.
Wedge pressure increased in both groups; however, this increase was only significant in the placebo-treated animals. Similarly, the LV dP/dtmax significantly decreased in the placebo-treated animals with HF, but this decrease was not significant in the sitagliptin-treated animals. Cardiac contractility (Ees) and LV–arterial coupling (Ees/Ea) significantly decreased in both groups. Left ventricular afterload (Ea) remained unchanged (Table 1).
The GFR remained preserved in the sitagliptin group in contrast to the placebo group where a significant reduction in GFR was noted after the 4 week period. The RPF decreased in both groups, although the decrease was more pronounced in the placebo compared with the sitagliptin group (Table 1).
Acute haemodynamic effects of brain natriuretic peptide infusion
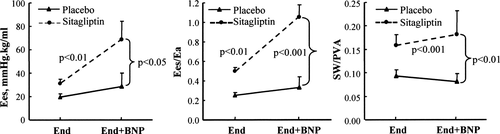
To determine whether sitagliptin treatment potentiates the effects of BNP on cardiac performance and contractility in HF, porcine BNP was administered at the end of the study protocol. In both groups, BNP exerted no effects on pulmonary artery or wedge pressures (data not shown). BNP infusion did not modify global cardiac performance (SV in the placebo group 1.43 ± 0.28 mL/kg before BNP vs. 1.45 ± 0.28 mL/kg after BNP, SV in the sitagliptin group 1.72 ± 0.13 mL/kg before BNP vs. 1.63 ± 0.15 mL/kg after BNP, HR in the placebo group 81 ± 1 b.p.m. before BNP vs. 89 ± 13 b.p.m. after BNP, HR in the sitagliptin group 60 ± 2 b.p.m. before BNP vs. 70 ± 9 b.p.m. after BNP). In contrast, BNP infusion resulted in an acute increase in LV contractility, ventricular–arterial coupling, and mechanical efficiency in the sitagliptin group, whereas no changes were observed in the placebo group (Figure 3). Interestingly, during BNP infusion, LV contractility, ventricular–arterial coupling, and mechanical efficiency returned almost to the levels seen at baseline in the sitagliptin-treated animals (Table 1 and Figure 3).
Effect of chronic dipeptidyl peptidase IV inhibition on tissue remodelling in heart failure
Body weight was not different between the two groups. A trend toward an increase in the heart weight to body weight ratio was obtained in the sitagliptin group compared with placebo (0.68 ± 0.04 vs. 0.57 ± 0.03%, P = 0.1). No white cell infiltration was found. Very little fibrous tissue was apparent and no difference in fibrosis density was detected between the control, placebo, and sitagliptin groups. Mean cross-sectional muscle fibre diameter was larger in the sitagliptin group than in the control group (52.8 ± 2.2 vs. 45.1 ± 2.0 μm, P < 0.05), while no difference was observed with the placebo group (50.6 ± 3.2 μm). A trend toward an increase in capillary density was observed in the sitagliptin group compared with the placebo group (1231 ± 103 vs. 792 ± 201 capillaries/mm2, P = 0.08). No difference for the control group was noted (1047 ± 61 capillaries/mm2).
Effect of chronic dipeptidyl peptidase IV inhibition upon myocardial gene expression in heart failure (Table 2)
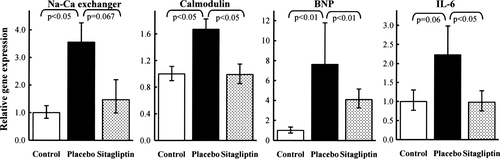
In both the placebo- and sitagliptin-treated animals, the presence of HF induced a myocardial down-regulation of the genes encoding SERCA and PLB compared with control pigs. In contrast, there was a significant up-regulation of NCX and CaM gene expression in the placebo group which remained unchanged compared with controls in the sitagliptin group (Figure 4). Myocardial BNP, IL-6, and MCP1 mRNAs were expressed at a higher level in placebo HF pigs than in controls and sitagliptin HF animals, while TNF-α and β1-AR stayed unchanged (Table 2, Figure 4). Myocardial SDF-1 mRNA tended to be up-regulated in HF sitagliptin-treated animals compared with controls. The LV SDF-1 protein level was higher in the sitagliptin group (180.3 ± 21.8 pg/mg total protein) than in the control group (132.1 ± 8.1 pg/mg total protein, P < 0.05). No significant change was noted for the placebo group (158.1 ± 3.1 pg/mg total protein).
| Genes | Control | Placebo | Sitagliptin | |||
|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | |
| SERCA | 1.00 | 0.88–1.14 | 0.61* | 0.58–0.64 | 0.59* | 0.52–0.67 |
| PLB | 1.00 | 0.90–1.11 | 0.41** | 0.35–0.48 | 0.49** | 0.43–0.55 |
| CaM | 1.00 | 0.90–1.11 | 1.67* | 1.53–1.83 | 0.99† | 0.85–1.15 |
| NCX | 1.00 | 0.80–1.25 | 3.56* | 2.98–4.25 | 1.47P =0.067 | 0.99–2.19 |
| β1-AR | 1.00 | 0.93–1.08 | 0.82 | 0.73–0.92 | 0.99 | 0.90–1.09 |
| BNP | 1.00 | 0.74–1.34 | 7.65** | 4.96–11.79 | 4.09†† | 3.25–5.15 |
| TNF-α | 1.00 | 0.76–1.31 | 1.08 | 0.88–1.33 | 1.01 | 0.75–1.36 |
| IL-6 | 1.00 | 0.77–1.30 | 2.23P =0.06 | 1.67–2.99 | 0.98† | 0.75–1.28 |
| MCP1 | 1.00 | 0.71–1.42 | 3.23* | 2.74–3.80 | 2.07† | 1.85–2.32 |
| SDF-1 | 1.00 | 0.78–1.27 | 1.09 | 0.83–1.43 | 1.64P =0.09 | 1.41–1.9 |
- a The range given for placebo and sitagliptin relative to control is determined by evaluating the expression: 2−ΔΔCT with ΔΔCT + SEM and ΔΔCT – SEM, where SEM = the standard error of the mean of the ΔΔCT value.
- * P < 0.05 and
- ** P < 0.01 vs. control
- † P < 0.05 and
- †† P < 0.01 vs. placebo; P =0.067 vs. placebo; P =0.06 vs. control; P =0.09 vs. control; Control, n = 6; placebo, n = 6; sitagliptin, n = 6.
- f β1-AR, β1 adrenoreceptor; BNP, brain natriuretic peptide; CaM, calmodulin; IL-6, interleukin-6; MCP1, monocyte chemotactic protein 1; NCX, Na+–Ca2+ exchanger; PLB, phospholamban; SERCA, sarcoplasmic endoplasmic reticulum calcium ATPase; SDF-1, stromal cell-derived factor 1; TNF-α, tumour necrosis factor-α .
Discussion
The present study is the first to evaluate the in vivo cardiorenal effects of DPP4 inhibition in a large animal model of HF. While DPP4 inhibition did not prevent cardiac dilation, cardiac myocytehypertrophy, and decline in the ejection fraction, the chronic administration of the DPP4 inhibitor sitagliptin reduced the HR and improved LV haemodynamics by increasing SV as well as maintaining LV dP/dtmax and filling pressure. In addition, it is noteworthy that treatment with this DPP4 inhibitor preserved the GFR, thereby preventing the development of cardiorenal syndrome. Finally, chronic treatment with sitagliptin restored the blunted cardiac response to BNP administration. Sitagliptin potentiated the positive inotropic effect of intravenous BNP administration by improving cardiac contractility, ventricular–arterial coupling, and mechanical efficiency.
As expected, 4 weeks of rapid pacing resulted in dilated cardiomyopathy characterized by LV dilation, systolic dysfunction, increased LV filling pressures, depressed contractility, ventricular–arterial uncoupling, impaired myocardial energetics, and renal insufficiency with impaired renal blood flow and glomerular filtration. The pigs were classified in stage B of HF.11
Pre-clinical data regarding chronic toxicity show that, in large animals, sitagliptin dosage up to 100 mg/kg is safe.12 Therefore, the dosage of 30 mg/kg once a day was selected to be sure to obtain a 24h inhibition of in vivo plasma DPP4 activity.
Our data demonstrate that although sitagliptin did not prevent the LV dilation and decline in the pump function, sitagliptin-treated pigs presented with a less severe dilation of the left atrium and no significant change in the wedge pressure, reflecting a better haemodynamic profile. Sitagliptin was associated with a relative increase in SV compared with the placebo group in parallel with a reduction of HR. The relative increase in SV and the well maintained dP/dtmax in the sitagliptin-treated animals are consistent with improved LV systolic function.
On one hand, this supports the hypothesis that sitagliptin inhibits the truncation of endogenous BNP1−32 to the less bioactive BNP form and as such increases the bioavailability of active BNP. On the other hand, alternative mechanisms should be considered. DPP4 is a pleiotropic enzyme that can truncate a variety of peptide hormones with potential haemodynamic effects such as glucagon like peptide-1(GLP-1), substance P (SP), SDF-1, and neuropeptide Y (NPY).13 Infusion of GLP-1 improves LV function in patients with severe HF14 while a decrease in HR and an increase in SV have been observed during GLP-1 infusion in a canine model of tachycardiomyopathy.15 However, as cardiac benefits are maintained when administering GLP9−36 instead of GLP7−36,16 there is no clear evidence for a role for GLP-1 in the sitagliptin effects. Very recently it has been demonstrated that an SP injection, producing no effect on cardiac function when given alone, attenuates norepinephrine chronotropic effects.17 From these studies, it appears that SP might be directly implicated in the decreased HR observed in HF pigs treated with sitagliptin. On the other hand, the decrease in HR may be due to the better haemodynamic profile associated with less sympathetic outflow. SDF-1 is another DPP4 substrate that, by attracting stem and progenitor cells, plays an important role in angiogenesis.18 In our study, the high SDF-1 myocardial levels obtained in sitagliptin-treated animals associated with increased capillary density compared with placebo may therefore participate in the cardioprotection induced by DPP4 inhibition. NPY, another DPP4 substrate, mediates vasoconstriction. The effects of DPP4 inhibition on arterial pressure are contradictory depending on the disease.19–21 However, to the best of our knowledge, our results are the first showing that DPP4 inhibition does not affect blood pressure in HF.
A similar decrease in Ees was observed in placebo- and sitagliptin-treated animals. This result is prima facie in discrepancy with a better systolic function in sitagliptin-treated pigs. However, the end-systolic pressure–volume relationship is known to be HR sensitive, with Ees increasing linearly in the interval between 60 and 120 b.p.m.22 In our study, the decreased HR in sitagliptin-treated animals is a confounding factor for the comparison of Ees in HF pigs.
Decline in renal function has a significant impact on outcomes in patients with HF and so the finding that sitagliptin can preserve GFR and limit decreases in RPF is appealing. This is consistent with a protective effect of DPP4 inhibitors on renal glomerular function during the development of HF, thereby disrupting the cascade that leads to cardiorenal syndrome. Whether this protective effect is related to enhanced renal availability of BNP1−32 or reflects a better haemodynamic profile is unclear. Several experimental studies have demonstrated that agents that potentiate its actions are renoprotective.23,24 Other findings demonstrated that DPP4 inhibition leads to alterations in the peptidome of DPP4-regulated proteins in the kidney.25 Therefore, in addition to the increase in the biological half-life of BNP, other factors may contribute to the observed renal protection by sitagliptin.
To address further whether chronic DPP4 inhibition may potentiate the haemodynamic effects of BNP, we administered a BNP infusion which acutely improved cardiac contractility and mechanical efficiency, and restored ventricular–arterial coupling in sitagliptin-treated pigs. The exact mechanism for these improvements remains unclear; however, it is most likely to be due to changes in intrinsic myocyte function induced by BNP. Downstream signalling of BNP in ventricular cardiac myocytes is production of cyclic guanylyl monophosphate (cGMP). In vitro data have demonstrated a biphasic effect of cGMP in cardiac myocytes, with a positive inotropic response at low levels and negative inotropic response at high levels.26 Although the in vivo, direct effects of BNP on intrinsic contractility are less clear, Lainchbury et al. have shown that a BNP infusion at a dose of 0.1 µg/kg/min increases Ees in healthy dogs but not in dogs with overt HF.27 Our findings corroborate those of this earlier observation. Indeed, no effect was observed in LV contractility during BNP infusion in the placebo-treated HF animals, whereas animals pre-treated with sitagliptin showed acute improvement in contractility. It remains to be seen if this positive inotropic response is explained by the fact that sitagliptin-treated pigs were in a less severe state of HF than the placebo-treated pigs or if DPP4 blockade increased the blood concentration of active BNP by inhibiting the cleavage of BNP1−32. Indeed, by measuring estimated in vivo DPP4 inhibition in sitagliptin-treated animals and showing a modest decrease in glycaemia, we proved sitagliptin activity in pigs as well as the metabolic effect associated with DPP4 inhibition; despite this, body weight was not different between the two groups. However, because of the lack of high affinity antibodies against porcine BNP, BNP fragments could not be detected. Nevertheless, the direct and important consequence was that mechanical efficiency and ventricular–arterial coupling approximated baseline values during BNP administration in the sitagliptin group.
We further explored whether chronic sitagliptin treatment was associated with changes in the molecular fingerprint of HF. We found, as expected, that in HF pigs, SERCA and PLB gene expressionwas reduced, whereas NCX and CaM mRNA levels were increased.28 In contrast to interventions with beta-blockers29, rosuvastatin,30 or cardiac resynchronization therapy,31 sitagliptin treatment failed to restore the mRNA levels of SERCA or PLB. Nevertheless, LV NCX and CaM mRNA levels in the sitagliptin group were comparable with those of controls. This finding might have clinical implications. NCX is rapidly up-regulated at the transcript and protein levels in models of HF32 and in human end-stage HF.33 A shift in the SERCA–NCX balance may play a major role in the contractile dysfunction observed in HF by causing a shift in calcium cycling toward more calcium cellular extrusion and less sarcoplasmic reticulum calcium uptake and release.34 Moreover, overactivity of NCX increases the propensity for triggered arrhythmias in HF so that NCX up-regulation appears to be a critical link between contractile dysfunction and arrhythmogenesis.35
BNP myocardial gene expression is stimulated in HF by increased cell stretch and neurohormonal activation. It is a marker of ventricular overload or cardiac remodellling, or it can indicate a transition from compensated to decompensated cardiac hypertrophy.36 BNP mRNA levels were higher in HF pigs compared with controls. Sitagliptin treatment decreased BNP expression. This may be related to a better haemodynamic profile and lower wall stress, or a regulatory negative feedback mechanism triggered by the availability of the active peptide.
Finally, IL-6, one of the cytokines known to be increased in HF and reduced after treatment,30 was expressed at a higher level in placebo-treated HF pigs than in sitagliptin-treated HF pigs. The production of IL-6, but not TNF-α, by stimulated lymphocytes has already been reported to be decreased upon DPP4 inhibition.37 Further studies are needed to unravel the role of DPP4 inhibitors in the genotypic fingerprint of HF.
Conclusion and clinical implications
This study reports for the first time that in experimental HF, DPP4 inhibition may help to preserve glomerular function, increase SV, and decrease HR. More substantially, co-administration of exogenous BNP during DPP4 inhibition significantly improves cardiac contractility, mechanical efficiency, and ventricular–arterial coupling, whereas no effect of BNP infusion alone is observed. Functional benefits are associated with less alteration up to normalization in myocardial gene expression of distinct calcium regulatory proteins, BNP, and IL-6, and with an increase in SDF-1. Whether this therapeutic strategy originally designed to overcome insulin resistance improves cardiac performance in diabetic patients with concomitant LV dysfunction remains to be seen.38 These novel findings provide insights into the role of DPP4 inhibition and warrant further studies to better understand its ability to prevent or treat the cardiorenal syndrome in HF.39
Supplementary material
Supplementary material is available at European Journal of Heart Failure online.
Acknowledgements
The excellent technical assistance of Pascale Jespers is greatly appreciated.
Funding
Biosite Incorporated, San Diego, USA; the Foundation for Cardiac Surgery, Brussels, Belgium; the Research Foundation – Flanders, Brussels, Belgium (FWO Vlaanderen, grant no. G016209). V.M. is a research assistant of FWO Vlaanderen; the Colombian Government to N.G.; the Erasme Foundation to K.T.; the Ministry of Health and Medical Education, Iran to M.M.
Conflict of interest: none declared.




