A new mechanism preventing proarrhythmia in chronic heart failure: rapid phase-III repolarization explains the low proarrhythmic potential of amiodarone in contrast to sotalol in a model of pacing-induced heart failure
Abstract
Aims
Life-threatening arrhythmias are a major problem in chronic heart failure (CHF). The aim of the present study was to investigate the mechanism underlying the low proarrhythmic potential of amiodarone in a model of pacing-induced heart failure.
Methods and results
Heart failure was induced in 35 female rabbits by rapid ventricular pacing, resulting in a significant decrease of ejection fraction. Thirty-four rabbits were sham-operated. In 17 of 35 CHF-rabbits and 20 of 34 ‘sham’-rabbits, amiodarone (50 mg/kg/day) was fed for 6 weeks. Eight monophasic action potentials and a simultaneously recorded 12-lead electrocardiogram showed prolongation of QT-interval and action potential duration (APD90) in all failing hearts (P< 0.05). Amiodarone pre-treatment led to a prolongation of APD90 (+19 ms) as compared with sham-controlled hearts but showed only a marginal effect on APD90 in failing hearts. Infusion of sotalol (50–100 µM) led to a significant prolongation of APD90 in sham and a further prolongation of APD90 in failing hearts [+55 ms (50 µM); +70 ms (100 µM); P< 0.01 as compared with sham hearts]. Sotalol led to a triangular action potential configuration in sham and failing hearts, whereas amiodarone did not cause triangularization but caused a rapid phase-III repolarization. Moreover, amiodarone did not increase dispersion of repolarization either in sham or in failing hearts. Infusion of sotalol led to a significant increase in dispersion of repolarization in sham (+29 ms) and a further increase in failing hearts (+67 ms; P< 0.05).
Conclusion
Chronic amiodarone results in a rapid phase-III-repolarization and does not increase dispersion of repolarization. These electrophysiological findings are present in healthy hearts and are preserved in heart failure. This contributes to the low proarrhythmic potential of amiodarone in heart failure.
Introduction
Chronic heart failure (CHF) can be regarded as a clinical presentation of different cardiovascular disorders. In CHF, ventricular arrhythmias are a frequent finding and CHF is a major underlying condition which is correlated to sudden cardiac death (SCD).1 In patients with CHF, the risk of SCD is 6–9 times higher than in the general population. Up to 50% of these patients die unexpectedly in the early stages of heart failure but there is no good correlation between the severity of heart failure and the incidence of SCD.2 Most SCDs follow ventricular tachyarrhythmias.3
Chronic heart failure leads to down-regulation of potassium channels4 resulting in a significant prolongation of the QT-interval as a consequence of a prolonged repolarization process. Thus, CHF can be considered as a mild form of acquired long QT syndrome, and an increased prevalence of potentially life-threatening ventricular tachycardia of the torsade de pointes (TdP) type has been observed in the clinical scenario of heart failure.5 In addition, in patients suffering from heart failure, concomitant drug therapy with repolarization prolonging drugs may contribute to proarrhythmia. Moreover, individuals with CHF, who subsequently develop arrhythmias, such as atrial fibrillation, may become more symptomatic and require antiarrhythmic drug therapy. Thus, the repolarization reserve might be further diminished by medication that affects the delayed rectifier potassium current IKr6 which may increase the risk of life-threatening arrhythmias. Additional proarrhythmic risk factors such as hypomagnesaemia, hypokalaemia, bradycardia, or drug accumulation due to liver or renal failure might further stress the repolarization reserve.
Amiodarone is one of the few remaining treatment options to treat ventricular arrhythmias and to reduce the incidence of atrial fibrillation without increasing mortality or SCD rates in patients with CHF.7 Due to an increasing prevalence of CHF, there is a growing need for antiarrhythmic agents to prevent frequent appropriate discharges of implantable defibrillators. Amiodarone has been shown to reduce the burden of arrhythmic events and ICD-shocks.8 In addition, amiodarone plays a major role in the treatment of atrial fibrillation, especially in heart failure with severely impaired left ventricular function, where class-I antiarrhythmic drugs or dronedarone are considered as contra-indicated.9,10 Interestingly, amiodarone does not seem to increase the risk of proarrhythmia or SCD despite marked QT-prolongation (<0.5 to >5%).11 Although amiodarone blocks multiple ion currents in the heart,12 the electrophysiological effects by which amiodarone exerts its strong antiarrhythmic action and its low proarrhythmic effects are not well understood. We have recently demonstrated that amiodarone has a low proarrhythmic potential in normal hearts due to a fast phase-III repolarization, a weak effect on dispersion of repolarization, a lower potential to induce early after depolarizations (EAD) and a weaker effect on reverse-frequency dependence as compared with sotalol.13 The aim of the present study was to elucidate whether these electrophysiological mechanisms also play a role in CHF.
Methods
All experimental protocols were approved by the local animal care committee and conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 852-3, revised 1996).
Induction of heart failure
Heart failure was induced via 3 to 4 weeks of ventricular pacing at 400 bpm. Adult female New Zealand white rabbits were anaesthetized with ketamine (75 mg/kg) and xylazine (5.8 mg/kg), with additional doses applied as needed. A pacemaker lead for transient pacing in the clinical setting was implanted into the right ventricle via the right jugular vein under fluoroscopic guidance. After testing the pacing threshold, the wounds were closed. One week after the operation, an external pacemaker was connected and pacing started at 400 bpm at twice the diastolic threshold. Over a period of 3 to 4 weeks, the rabbits were monitored by clinical examination and ECG to observe whether they developed CHF.14 In addition, sham-operated rabbits served as controls.
Preparation of hearts for perfusion
Thirty-seven white female New Zealand rabbits (weighing 3.5–4.0 kg) received oral amiodarone treatment (50 mg/kg body weight by mouth per day) for 6 weeks. It has previously been shown by our group in the same model that this dosage prevents ventricular tachycardias and ventricular fibrillation, and that achieved tissue levels correspond to human tissue levels under amiodarone treatment (6.5 ± 0.9 to 11.9 ± 2.3 μg in this study).15 The drug was mixed into the normal food and application started 3 weeks prior to surgery. Then, 17 of these 37 rabbits (randomly assigned) received a pacemaker as described above while the remaining 20 rabbits were sham-operated and observed. Amiodarone was fed continuously until the Langendorff experiment was performed. In addition, 18 untreated rabbits of comparable weight also received a pacemaker while 14 untreated rabbits were sham-operated. These animals served as ‘baseline’-controls for the amiodarone group before their hearts were infused with sotalol later on.
The method of preparing the hearts has previously been described in detail.16 In short, female New Zealand white rabbits were anaesthetized with sodium thiopental (200–300 mg intravenously). The complete hearts were removed and immediately placed in an ice-cold Krebs–Henseleit solution (composition in mM: CaCl2 1.80, KCl 4.70, KH2PO4 1.18, MgSO4 0.83, NaCl 118, NaHCO3 24.88, Na-pyruvate 2.0, and d-glucose 5.55). The spontaneously beating hearts were perfused at constant flow (52 mL/min) with warm (36.8–37.2°C) Krebs–Henseleit solution. Perfusion pressure was kept stable at 100 mmHg. The perfusate was equilibrated with 95% O2 and 5% CO2 (pH 7.35; 37°C). The cannulated and perfused hearts were attached to a vertical Langendorff apparatus (Hugo Sachs Electronic, Medical Research Instrumentation, March-Hugstetten, Germany). A deflated latex balloon was inserted into the left ventricle and connected to a pressure transducer to control haemodynamic stability. The atrioventricular (AV) node was ablated. This resulted in complete AV-dissociation with a ventricular escape rate below 60 bpm.
Electrocardiographic and electrophysiological measurements
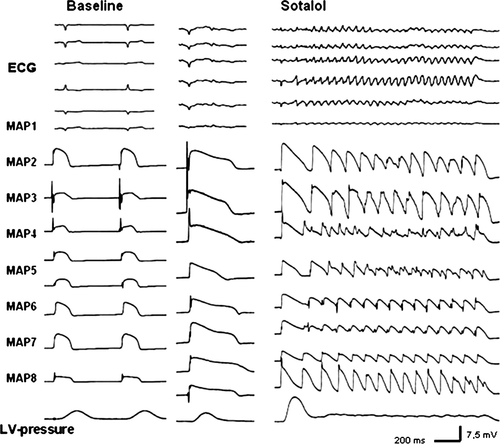
Signals from a simulated ‘Einthoven’ configuration were amplified by a standard ECG amplifier (filter settings: 0.1–300 Hz), and QT-interval was measured. Monophasic action potential (MAP) recordings and stimulation were accomplished simultaneously using contact MAP-pacing catheters (EP Technologies, Mountain View, CA, USA). The MAP electrograms were amplified and filtered (low pass 0.1 Hz, high pass 300 Hz). Monophasic action potentials were analysed using specifically designed software, permitting precise definition of the amplitude and duration of the digitized signals. The recordings were considered reproducible and, therefore, acceptable for analysis only if they had a stable baseline amplitude with a variation of less than 20% and a stable duration measured at 90% repolarization (APD90). Seven MAPs were evenly spread in a circular pattern around both ventricles; one MAP was recorded from the left endocardium (Figure 2). Pacing at twice diastolic threshold was performed for 1 min at each cycle length (CL) from 900 to 300 ms (Universal Programmable Stimulator, UHS 20, Biotronik, Germany). All data were digitized at a rate of 1 kHz with 12-bit resolution and subsequently stored on a removable hard disk (BARD LabSystem, Bard Electrophysiology, Murray Hill, MA, USA).
Experimental ex vivo protocol
Cycle length dependence of MAPs was investigated under baseline conditions in amiodarone-treated hearts and in the control group by pacing the hearts at CLs between 900 and 300 ms. Subsequently, programmed stimulation was performed with up to two extrastimuli (increment of 2 ms) to determine the refractory period. Thereafter, the potassium concentration was lowered to 1.5 mM to provoke EADs and TdP in spontaneously beating hearts with their slow ventricular escape rate. Five minutes later, the potassium concentration was set back to 5.8 mM and the control hearts were perfused with d,l-sotalol in increasing concentrations (50–100 µM) over a period of 20 minutes. The experimental set-up was designed to reproduce conditions and circumstances that are clinically known to be associated with an increased propensity to the development of TdP. Pacing, MAP recording, and measurement of ECG parameters were started 20 min after drug infusion. The protocol steps were repeated for each drug concentration. In summary, data were obtained from CHF-hearts and control hearts, both chronically treated with amiodarone, and CHF-hearts and control hearts at baseline and after perfusion with 50 and 100 µM sotalol.
APD90 was measured as the average interval between the fastest MAP upstroke and 90% repolarization. Dispersion of APD90 was expressed as the difference between the maximum and the minimum of APD90, simultaneously recorded in eight endocardial and epicardial catheters. Monophasic action potential morphology was assessed by calculating the ratio of APD90/50, thereby quantifying a triangular or rectangular action potential shape. Early afterdepolarizations were defined as a positive voltage deflection that interrupted the smooth contour of phase-II or III repolarization of the action potential. Torsade de pointes was defined as a polymorphic ventricular tachyarrhythmia of more than five beats with a changing ventricular axis and spontaneous termination.
Data acquisition and statistical analysis
The observed data were entered into a computerized database (Microsoft Excel 2003) and statistically evaluated using SPSS Software Release 15.0.1 (November 2006, Chicago). Categorical variables were expressed as frequency and percentage, whereas continuous variables were presented as mean ± SD. The effect of sotalol and amiodarone on CL dependence QT-interval, action potential duration, and dispersion of repolarization were assessed using general linear model for repeated measurements. After confirming significant group differences over CL, post hoc comparisons with Tukey's procedure were applied to determine differences between groups. McNemar tests were used to compare the appearance of EADs and TdP. The χ2 test and Fisher's test were used to compare TdP incidence. T-tests were used to compare the difference between two independent study groups, and the paired-samples t-test between dependent study groups. Differences were considered significant at P< 0.05.
Results
Assessment of heart failure
During the period of fast ventricular pacing, rabbits became lethargic, fatigued, showed loss of appetite, and developed respiratory distress. Moreover, the paced rabbits developed ascites, pleural and pericardial effusion, and peripheral oedema. To monitor the progress of left ventricular (LV)-function impairment, two-dimensional ECG was carried out periodically and demonstrated a significant decrease in ejection fraction from 74±6–22±8% in the presence of a dilated LV after 3 to 4 weeks. After removal, the failing hearts were found to have a significantly greater LV-diameter (mean—left ventricular enddiastolic diameter: 1.86 ± 0.18 cm in heart failure as compared with 1.64 ± 0.14 cm in control hearts, P< 0.05), and increased weight (mean 19.3 ± 2.7 g in heart failure as compared with 16.5 ± 2.1 g in controls; P<0.05). Histological examination showed an increase of perivascular and interstitial fibrosis. The comparison of baseline electrophysiologic values of intact and failing hearts revealed a significant prolongation of QT-interval and action potential duration (Figure 1A).
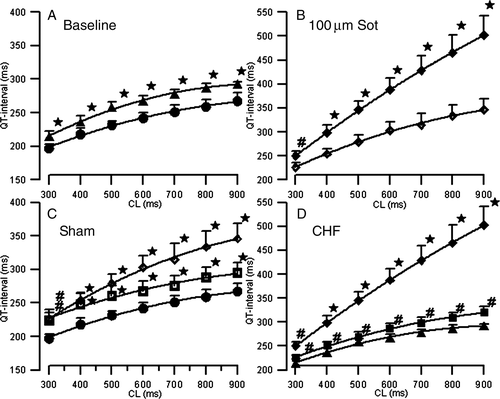
Effect of sotalol on QT-interval, action potential duration, and dispersion of repolarization in sham and failing hearts
After a stabilization period of 10 min, MAP recordings and pacing thresholds (mean threshold 1.6 ± 1.2 mA) remained highly reproducible throughout the experimental protocol. The MAP amplitude did not change by more than 20% for the subsequent investigation period. Sotalol (50 and 100 µM) in sham-operated (n= 14) and failing (n= 18) hearts led to a significant increase in QT-interval and monophasic action potential duration (APD90; P< 0.05), thereby mimicking LQT syndrome. Figure 1B and C illustrate the effects of sotalol on QT-interval. In the presence of 100 µM sotalol, the QT-interval increase ranged between 15% at a CL of 300 ms and 29% at a CL of 900 ms (P< 0.05) in sham hearts. This so-called reverse-frequency dependence17 was even more marked in failing hearts (17–72%; P< 0.05) representing a reduced repolarization reserve in heart failure (Figure 1D). The increase in QT-interval by sotalol was paralleled by a significant increase in APD90. Up to eight simultaneous epi- and endocardial MAPs demonstrated a significant increase in dispersion of repolarization in sotalol-treated hearts from baseline values of 41–70 ms in sham hearts. Again, the increase in dispersion of repolarization was more marked in failing hearts due to a reduced repolarization reserve (35–102 ms in failing hearts after drug infusion; Figure 2). Dispersion of repolarization was markedly increased at slow heart rates (Table 1) and resulted from an increase in interventricular (recorded between right epicardium and left epicardium (basal and apical MAP recordings) and transmural (recorded between left endocardium and left epicardium) dispersion.
| CL | Sotalol—Sham baseline | Sotalol—Sham 100 µM | Sotalol—CHF Baseline | Sotalol—CHF 100 µM |
|---|---|---|---|---|
| 900 | 38 ± 11 | 81 ± 31 | 38 ± 10 | 152 ± 72 |
| 800 | 39 ± 10 | 78 ± 24 | 41 ± 14 | 142 ± 58 |
| 700 | 40 ± 14 | 77 ± 23 | 38 ± 12 | 115 ± 41 |
| 600 | 41 ± 10 | 71 ± 20 | 34 ± 11 | 97 ± 31 |
| 500 | 42 ± 11 | 66 ± 16 | 40 ± 10 | 83 ± 28 |
| 400 | 42 ± 15 | 60 ± 15 | 39 ± 17 | 69 ± 23 |
| 300 | 42 ± 13 | 56 ± 16 | 37 ± 12 | 58 ± 18 |
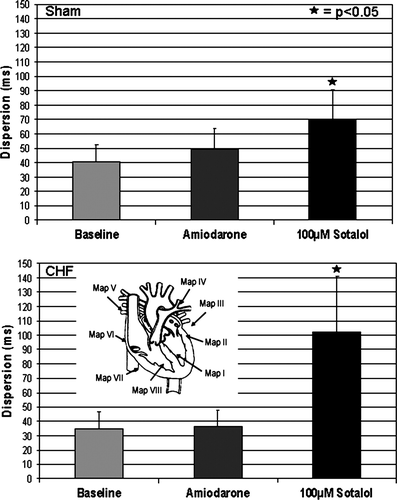
Effect of amiodarone on QT-interval, action potential duration, and dispersion of repolarization
In sham hearts, chronic administration of amiodarone led to a prolonged QT-interval as compared with untreated control hearts (271 –238 ms). The prolonged QT-interval was accompanied by a significant increase of APD90. In contrast to sotalol, no reverse-frequency dependence (14% increase at a CL of 300 ms, 10% at 900 ms, Figure 1C) and no significant increase in dispersion of repolarization could be observed (Figure 2 top). In failing amiodarone-treated hearts, only a non-significant rise in QT-interval (271–280 ms; Figure 1D) and APD90 was observed. Again, there was no increase in dispersion of repolarization (Figure 2, bottom) compared with failing baseline hearts.
Refractoriness and action potential configuration
A comparison between sham and failing hearts at baseline revealed an increase in the refractory period from 173 to 199 ms. The administration of both sotalol and amiodarone augmented the refractory period in control hearts from 173 to 199 ms (amiodarone) and from 173 to 222 ms (100 µM sotalol). In failing hearts, sotalol increased the refractory period from 199 to 291 ms. Comparable with APD90, amiodarone hearts did not show a significant increase.
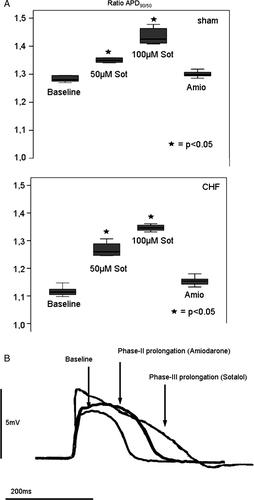
Sotalol led to a triangular action potential configuration (ratio APD90/APD50 sham: 1.29 at baseline, 1.44 at 100 µM sotalol; CHF: 1.11 at baseline, 1.35 at 100 µM sotalol; Figures 3A and 3B), whereas amiodarone did not cause triangularization but caused a more marked phase-II prolongation (APD90/APD50 sham: 1.29 at baseline in controls, 1.30 after treatment with amiodarone; CHF: 1.11 at baseline in controls, 1.15 after treatment with amiodarone; Figures 3A and 3B).
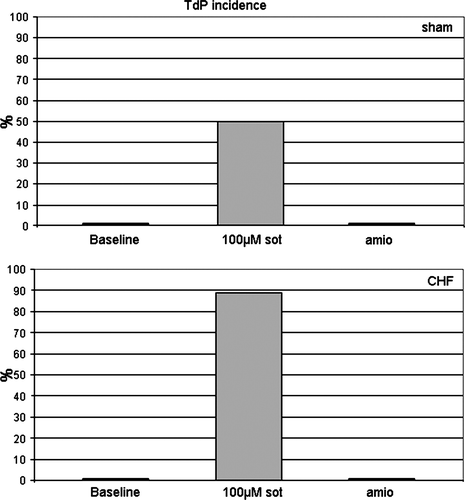
Early afterdepolarizations and torsade de pointes
In the presence of sotalol, EADs and triggered activity were a frequent finding. After complete AV-block and lowering of potassium concentration from 5.88 to 1.5 mM/l, 7 of 14 sotalol-treated sham hearts showed MAP recordings with EADs and TdP (50%, 35 single episodes (Figure 4). A typical example during sotalol 100 µM in a CHF heart is shown in Figure 5. Torsade de pointes were always associated with EADs [sotalol: P< 0.05 (Fisher-test)]. In failing sotalol-treated hearts, TdP could reproducibly be observed in 16 out of 18 hearts (Figure 4; 89%, 154 single episodes). In amiodarone-treated sham and failing hearts, no EADs or TdP could be observed.
Discussion
The main finding of the present study is that a fast phase-III repolarization after treatment with amiodarone was associated with a low proarrhythmic potential in heart failure. Despite diminished repolarization reserve in CHF, amiodarone had no effect on dispersion of repolarization, neither induced EADs nor demonstrated reverse-frequency dependence.
Extent of myocardial repolarization
In failing hearts, there was a significant prolongation of QT-interval and action potential duration as compared with non-failing hearts. Action potential prolongation, mainly due to deceleration of phase-III-repolarization, served as the key electrophysiological factor for the formation of triggered activity and potentially life-threatening arrhythmias. In heart failure, this results from a reduction in outward, repolarizing ion currents, especially due to down-regulation of potassium channels.18 Thus, CHF leads to a reduced ‘repolarization reserve’, which means a patient-specific response to risk factors that influence the repolarization process.19 Additional administration of agents that prolong repolarization may be harmful. The major antiarrhythmic mechanism of class-III drugs is prolongation of action potential duration, which unfortunately is also the mechanism underlying its proarrhythmic potential.6 However, despite comparable QT-prolongation, different cardiovascular and non-cardiovascular drugs, including the antiarrhythmic agents sotalol and amiodarone have a different proarrhythmic potential. Thus, prolongation of repolarization may act as a ‘conditio sine qua non’ for the occurrence of this type of proarrhythmia, but the correlation between the extent of QT-prolongation and the incidence of TdP is poor.20
Action potential morphology
Our group has previously shown in healthy rabbit hearts that differences in action potential morphology, different effects on dispersion of repolarization, and differences in reverse-frequency dependence contribute to the low torsadogenic potential of amiodarone.13 The present study showed for the first time, that comparable mechanisms play a role in non-ischaemic heart failure. Differences in the morphology of the action potential (rectangular vs. triangular) are associated with a divergent potential of a great variety of repolarization-prolonging drugs to provoke proarrhythmia. The proarrhythmic potential of a triangular action potential configuration was first presented by Hondeghem et al.21 Our group and others have also reported that the configuration of a prolonged action potential is a relevant factor for the proarrhythmogenic potential of QT-prolonging drugs.20 Moreover, we recently showed in healthy rabbit hearts ex vivo, that a rapid phase-III repolarization caused by amiodarone, leading to a rectangular action potential, is associated with a low proarrhythmic potential.13 In contrast, sotalol was found to prolong phase-III of the repolarization process. This marked prolongation enhanced the origination of EADs by creating more time in the voltage window for calcium channel reactivation as compared to amiodarone22 in the present study. By prolonging action potential duration within the L-type Ca2+ ‘window’ voltage range, more calcium can enter the cell and the Na+/Ca2+ exchanger is activated. This leads to an increased inward current that could redepolarize the sarcolemmal membrane to a potential from where INa and/or ICa becomes reactivated.23 The resulting inward current may depolarize the sarcolemmal membrane to a potential from where a new and premature action potential can be triggered.24 Besides, triangulation is capable of prolonging the recovery of the sodium current, which lengthens the vulnerable period.17 In contrast, in our model of normal and failing hearts, amiodarone did not cause triangulation but a rectangular action potential configuration due to a more marked phase-II prolongation by taking advantage of its effects on multiple ion channels, in this case especially inhibition of ICal. Compared with sotalol, in the setting of the failing rabbit heart amiodarone enhanced a fast and better synchronized repolarization that was less vulnerable to proarrhythmia.
Trigger and substrate for proarrhythmia
In non-ischaemic heart failure, triggered activity is a crucial mechanism for the origination of arrhythmias in the experimental25 and the clinical setting.26 In our study, the appearance of proarrhythmia was always associated with the occurrence of EADs and an increased dispersion of repolarization. The importance of EADs, especially in CHF, is underlined by the work of Pogwizd et al.26 in human failing hearts prior to explantation. They reported that focal initiation of ventricular tachycardia in heart failure was related to triggered activity initiated in the subendocardium of non-ischaemic dilated cardiomyopathy. In addition, an increased dispersion of repolarization is considered as the responsible substrate for the perpetuation of arrhythmias in the setting of proarrhythmia. It has been shown in experimental models and in humans that differences in the degree of increasing dispersion of repolarization contribute to a divergent proarrhythmic potential of many drugs.16,27 In the present study, the importance of dispersion of repolarization as the underlying substrate for proarrhythmia in CHF was measured by several simultaneously recorded MAPs. Using epicardial and endocardial MAP recordings, differences in action potentials recorded from different layers of myocardium were evident. The observed minor increase of dispersion by amiodarone compared with sotalol contributes to the lack of proarrhythmia. Shimizu et al.28 used MAP recordings in humans to support the hypothesis that proarrhythmia is maintained by a reentrant mechanism due to increased dispersion of repolarization. Following comparable results in different studies, dispersion has been added to the relevant factors for the initiation of proarrhythmia.29 One underlying mechanism for the increase in dispersion of repolarization is a heterogenous connexin-43-expression that has been identified as a relevant factor that increases vulnerability to ventricular tachyarrhythmias in CHF.30 Moreover, a significant decrease of connexin-43-expression has been reported in an animal model of tachycardiomyopathy.31 The decrease of the major gap junction protein leads to an uncoupling between muscle layers resulting in an increase of dispersion of repolarization that may facilitate the occurrence of ventricular tachyarrhythmias. In addition, in the context of CHF, many positive inotropic agents, used in treatment of end-stage heart failure, increase dispersion of repolarization.32 This leads to proarrhythmia, thus increasing mortality.33 Amiodarone has been shown to attenuate the proarrhythmic effects of dobutamine in patients with advanced heart failure,34 probably by creating a homogeneous repolarization.
Reverse-frequency dependence is regarded as a risk factor for the development of TdP.35 In sham hearts and even more pronounced in failing hearts, treated with sotalol, we observed a more marked prolongation of action potential duration at slower than at faster heart rates. This finding contributes to proarrhythmia occurring in CHF, because the higher increase of prolongation at slow rates leads to a marked increase of dispersion of repolarization (Table 1), thus unmasking a reduced repolarization reserve in CHF. In addition, the increased dispersion of repolarization at slower heart rates explains why bradycardia is a contributing factor for the occurrence of proarrhythmia. Neither failing nor intact amiodarone hearts showed reverse-frequency dependence. The lack of a remarkable effect on dispersion of repolarization during bradycardia contributes to the understanding of the low proarrhythmic potential of amiodarone.
Limitations of the study
Our experimental study was performed in isolated rabbit hearts and, therefore exact extrapolation of our experimental results to the human heart may be difficult; however, many studies show that the electrophysiological findings in rabbit hearts are comparable with observations in humans. Although the contribution of ion currents differs, the rabbit heart model seems to be appropriate for studying the mechanisms of repolarization and gives information about electrophysiological effects at the level of the whole heart, including cell-to-cell coupling effects. This is of particular interest with regard to dispersion of repolarization; however, data at the cellular or ion channel level would provide supplemental information. In addition, a model of pacing-induced heart failure was employed to examine conditions of CHF and the results may be different in heart failure of a different origin.
Conclusion
The present data demonstrate for the first time that a fast phase-III repolarization was associated with a low proarrhythmic potential in CHF, whereas a more marked phase-III prolongation led to proarrhythmia. Moreover, use of amiodarone in CHF did not increase dispersion of repolarization or induce EAD or demonstrate reverse-frequency dependence. These electrophysiological properties of amiodarone have already been described in normal hearts. The present study clearly demonstrates that they also play an important role in failing hearts. This qualifies amiodarone for additional therapy in patients with end-stage heart failure, dependent on positive inotropic drugs, where proarrhythmia may occur. Currently, there is no alternative to amiodarone for antiarrhythmic drug treatment in these patients. The role of dronedarone, which has a comparable electrophysiological profile, is still under debate in CHF. No increase in mortality and a reduction of cardiovascular hospitalization or death after dronedarone treatment was reported for patients with atrial fibrillation and stable CHF in the ATHENA study.36 However, there is a contraindication for patients with severe heart failure since dronedarone leads to a worsening of heart failure.37 Other novel drugs such as ranolazine might have a comparable antiarrhythmic potential, but still need to be further investigated in CHF. The future development process of safer antiarrhythmic drugs should focus on action potential morphology as an important determinant of proarrhythmia.
Acknowledgements
We thank Irina Potthoff, MTA, for excellent technical assistance.
Funding
This study was supported by the Dr Peter Osypka Foundation, Lörrach. L.E. holds the Peter Osypka Professorship of Experimental and Clinical Electrophysiology.
Conflict of interest: P.M. has received lecture honoraria from sanofi-aventis. G.B. is member of scientific advisory boards of sanofi-aventis and MSD, and has received fees for lectures at symposia organized by sanofi-aventis. In addition, G.B. is a member of the Steering Committee of an international investigator-initiated trial of treatment strategies in atrial fibrillation for which sanofi-aventis is a sponsor. L.E. has received lecture honoraria from AstraZeneca, Biosense Webster, Biotronik, Boehringer Mannheim, Guidant, Medtronic, sanofi-aventis, and St Jude Medical, and research grants from Biotronik, St Jude Medical, Sanofi, and Osypka. The remaining authors have no conflicts of interest.




