Comparison of acute and chronic impact of adaptive servo-ventilation on left chamber geometry and function in patients with chronic heart failure
Abstract
Aims
The aim of this study was to determine differences in the acute and chronic impact of adaptive servo-ventilation (ASV) on left chamber geometry and function in patients with chronic heart failure (CHF).
Methods and results
An acute ASV study was performed to measure echocardiographic parameters before and 30 min after the initiation of ASV therapy in 30 CHF patients (mean age: 69 years, 23 male). The chronic effects of ASV therapy were also evaluated in 26 of these 30 patients over a mean follow-up period of 24 weeks. Patients were divided into two groups according to the status of ASV therapy [ASV group (n= 15) and withdrawal group (n= 11)]. In the acute study, heart rate and blood pressure were significantly decreased 30 min after the ASV therapy compared with baseline. Stroke volume and cardiac output were significantly increased in conjunction with a reduction in systemic vascular resistance. Multivariate regression analysis revealed baseline E/e′ to be an independent predictor for absolute increase in cardiac output. In the chronic study, a significant reduction of left ventricular (LV)/left atrial (LA) volumes and the severity of mitral regurgitation (MR), and improved LV diastolic function parameters were noted in the ASV group. These beneficial effects were not observed in the withdrawal group.
Conclusion
The acute beneficial impact of ASV is mainly associated with the reduction of afterload resulting in an increase in stroke volume and cardiac output. In contrast, chronic ASV therapy produces LV and LA reverse remodelling resulting in an improvement in LV function and the severity of MR in patients with CHF.
See page 1049 for the editorial comment on this article (doi:10.1093/eurjhf/hfr118)
Introduction
Chronic heart failure (CHF) is the final phase of cardiovascular disease, and is associated with high mortality and poor prognosis. Chronic heart failure is often accompanied by sleep-disordered breathing (SDB),1 and the frequency of central sleep apnoea with Cheyne–Stokes respiration (CSA–CSR) increases according to the severity of heart failure.2,3 Recently, treatment of SDB has emerged as an important therapeutic target in patients with advanced heart failure.4 Although positive airway pressure (PAP) therapies such as continuous positive airway pressure (CPAP) or bi-level PAP therapy are important therapeutic options in severe heart failure,5,6 there are some patients who are resistant to these devices for treating CSA–CSR.7,8
Adaptive servo-ventilation (ASV) is a novel PAP therapy, which was designed specifically for treatment of CSA–CSR.9 The ASV device is usually set at low-level of end-expiratory pressure (EEP), and provides automatically adjusted inspiratory pressure support for each breath. For this algorithm, it has been reported that ASV therapy significantly eliminates any type of SDB and produces larger improvements in left ventricular ejection fraction (LVEF) than CPAP therapy in CHF patients.10,11 However, the acute beneficial impact of ASV therapy on cardiac function and haemodynamics in patients with CHF has not been established. Moreover, there are minimal data regarding the effect of chronic ASV therapy on left chamber geometry and mitral regurgitation (MR). We hypothesized that the beneficial impact of ASV therapy on left ventricular (LV) and left atrial (LA) geometry and function and MR may be different between the acute and chronic phases. Accordingly, the aim of this study was to determine the effects of acute and chronic ASV therapy on haemodynamics, left chamber geometry and function, and MR in patients with CHF.
Methods
Study population
Thirty patients (mean age; 69 years, 23 male) with CHF (New York Heart Association functional class ≥II) and LV dysfunction [LVEF<50% on two-dimensional (2D) echocardiography], were included. Prior to inclusion in this study, all patients had been in a stable condition for >2 weeks. All patients were receiving optimal medical therapy with no changes in medication for at least 1 month. Patients who had received pacemaker, implantable cardioverter–defibrillator or cardiac resynchronization therapy devices were only enrolled if they were more than 6 months post-implantation. Exclusion criteria were: (i) obstructive sleep apnoea (obstructive apnoea index >5.0/h), (ii) acute decompensated heart failure or cardiogenic shock, (iii) chronic obstructive pulmonary disease, (iv) unstable angina and acute myocardial infarction, (v) pericardial and congenital heart disease, and (vi) previous use of PAP therapy. The Institutional Review Board of the University of Occupational and Environmental Health approved the study, and all patients gave informed consent prior to the study participation.
Cardio-respiratory polygraphy
To confirm that the patients had SDB and to exclude obstructive sleep apnoea, a sleep study was performed using cardio-respiratory polygraphy (Morpheus® R, Teijin Co. Tokyo, Japan) as previously described.12 Nasal airflow, chest and abdominal respiratory effort, and pulse oximetry were recorded continuously. Hypopnoeas were defined as a ≥30% reduction in airflow in combination with a drop in oxyhaemoglobin saturation of at least 4% from baseline values. Apnoeas were defined as a cessation of inspiratory airflow for at least 10 s, and were classified as either obstructive (if accompanied by typical thoraco-abdominal breathing effort) or central (if there was no effort). CSR is defined as typical crescendo–decrescendo ventilation with interposed central apnoea or periodic breathing (crescendo–decrescendo ventilation with intereposed hypopneoea). An apnoea−hypopnoea index (AHI) ≥15 h was considered as SDB.
Study protocol
Acute study
The acute ASV study was performed to determine the acute impact of ASV on cardiac function and haemodynamics. After 15 min of quiet rest on the bed in the echocardiography laboratory, measurements of heart rate, blood pressure and baseline 2D, and Doppler echocardiography examinations were performed. Then, ASV (Autoset™ CS®, ResMed, Sydney, Australia/Teijin CO. Tokyo, Japan) was applied using a full-face mask for default pressure settings (EEP: 5 cmH2O, inspiratory pressure support: 3–10 cmH2O). To evaluate the acute effects of ASV, haemodynamic measurements and the echocardiographic examination were repeated 30 min after the initiation of ASV therapy (Figure 1).
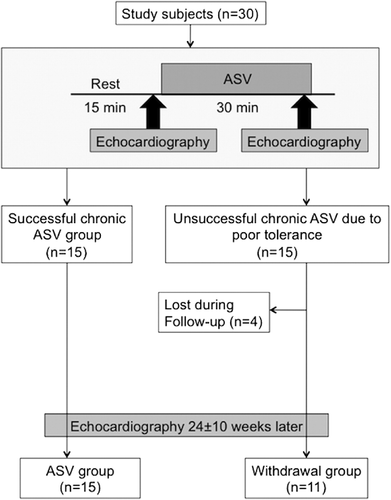
Chronic study
After the investigation of the acute effects of ASV, 15 patients refused to undergo chronic ASV therapy because of poor tolerance of PAP or wearing of the face mask. Consequently, the patients were divided into two groups; (i) 15 patients who continued ASV therapy for at least 4 h per day (ASV group), and (ii) 15 patients who did not continue ASV therapy (withdrawal group). Four patients in the withdrawal group were lost to follow-up. Thus, a total of 26 patients were included in the evaluation of chronic ASV therapy. The same echocardiographic parameters as for the acute study were obtained during the follow-up period (mean: 24 weeks). The pressure settings of ASV therapy and dosages of medications were not changed during the follow-up period.
Echocardiographic examination
Echocardiographic examinations were performed using a commercially available ultrasound machine (Vivid 7, GE Healthcare, Milwaukee, WI, USA) with a 3.5 MHz transducer. All images were acquired using second harmonic mode. Standard 2D images were obtained from parasternal and apical windows. In each plane, three consecutive cardiac cycles were acquired during a breath hold and digitally stored on a hard disk for off-line analysis. Left ventricular end-diastolic volume (LVEDV), end-systolic volume (LVESV) and LVEF were calculated using biplane Simpson's method from apical four-chamber and two-chamber views according to the recommendations of American Society of Echocardiography.13 Left atrial volume was calculated by the biplane area−length method from apical four-chamber and two-chamber views. Maximum and minimum LA volumes were measured. Pulsed Doppler sample volume was placed at the mitral valve tips in the apical four-chamber view to record LV inflow velocity. From LV inflow velocity, early diastolic peak flow velocity (E), late diastolic peak flow velocity (A), and deceleration time of the E wave velocity were measured. Tissue Doppler mitral annular velocity was recorded from the apical four-chamber view with the pulse-wave Doppler sample volume placed on the septal and lateral corners of the mitral annulus. Care was taken to ensure an ultrasound beam parallel to the direction of the mitral annular motion. Filters were set to exclude high-frequency signals, and gains were set to obtain clear tissue signals with minimal background noise. Peak systolic (s′), peak early (e′) diastolic, and late (a′) diastolic annular velocities were measured at each corner of the mitral annulus, and these values were averaged. Stroke volume was calculated as LV outflow tract area×velocity time integral of the LV outflow velocity. Cardiac output was obtained by stroke volume×heart rate. Vena contracta width was measured from parasternal or apical long-axis views to determine the severity of MR by 2D colour Doppler image. The width of the neck or narrowest portion of the MR jet was then measured.14 Systemic arterial compliance (SAC) was calculated as stroke volume divided by pulse pressure. Systemic vascular resistance (SVR) was calculated as (mean blood pressure/cardiac output)×80 as previously described.15
Inter- and intra-observer variability
Intra-observer variability was determined by having one observer repeat the measurement of LVEDV, LVESV, and maximum LA volume in 15 randomly selected patients 1 month apart. Inter-observer variability was determined by having a second observer measure these variables in the same datasets. Intra- and inter-observer variability values were calculated as the absolute difference between the corresponding two measurements as a percentage of the mean.
Statistical analysis
Continuous data are expressed as mean ± SD. Categorical data are presented as a number or percentage. Differences in continuous variables between two groups were evaluated using t-tests. Categorical variables were compared using Fisher's exact test or χ2 test as appropriate. Linear regression analysis was used to investigate the relation between two parameters. Univariate and multivariate liner regression analysis was performed to determine independent predictors for acute absolute increase of cardiac output in several haemodynamic and echocardiographic parameters including heart rate, systolic blood pressure, LVEDV, LVESV, and baseline E/e′. Similarly, univariate and multivariate regression analysis was also performed to determine independent predictors for chronic per cent reduction of LV or LA volumes in clinical, haemodynamic, echocardiographic parameters including baseline heart rate, E wave velocity, LVEDV, LVESV, maximal LA volume, E/e′, cardiac medication including angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, beta-blocker, calcium-channel blocker and diuretics and the usage of ASV. A P value of <0.05 was considered significant.
Results
Patient characteristics
Clinical characteristics and sleep study parameters of the study patients are shown in Table 1. The prevalence of moderate-to-severe SDB was 63%. Although the allocation to chronic ASV therapy was not randomized in this study, there were no significant differences regarding the duration of follow-up, patient characteristics, medication, or sleep study parameters between the ASV therapy group and the withdrawal group. Chronic ASV therapy produced a significant reduction in AHI from 25.3 ± 18.1 h in the acute study to 3.0 ± 2.3 h in the chronic study.
| All patients (n = 30) | ASV group (n = 15) | Withdrawal group (n = 11) | P value | |
|---|---|---|---|---|
| Age (years) | 69 ± 13 | 67 ± 11 | 67 ± 14 | NS |
| Gender (male/female) | 23/7 | 11/4 | 8/3 | NS |
| BSA (m2) | 1.57 ± 0.19 | 1.62 ± 0.14 | 1.56 ± 0.17 | NS |
| Ischaemic cardiomyopathy | 16 (53%) | 8 (53%) | 8 (73%) | NS |
| Comorbidities | ||||
| Hypertension | 19 (63%) | 8 (53%) | 9 (82%) | NS |
| Diabetes | 11 (37%) | 5 (33%) | 5 (45%) | NS |
| Atrial fibrillation | 9 (30%) | 5 (33%) | 4 (36%) | NS |
| Follow-up period (weeks) | 24 ± 10 | 24 ± 9 | 23 ± 12 | NS |
| Medications | ||||
| ACE-I/ARB | 28 (93%) | 15 (100%) | 11 (100%) | NS |
| β-blocker | 19 (63%) | 10 (67%) | 8 (73%) | NS |
| Ca-channel blocker | 7 (23%) | 2 (13%) | 3 (27%) | NS |
| Diuretics | 20 (67%) | 12 (80%) | 9 (82%) | NS |
| Statin | 11 (37%) | 5 (33%) | 5 (45%) | NS |
| Cardiac devices | ||||
| CRT-D/ICD/pacemaker | 3/3/2 | 2/1/1 | 1/2/1 | NS |
| Significant mitral regurgitation | 20 (77%) | 12 (80%) | 8 (73%) | NS |
| Sleep study parameters | ||||
| Apnea–hypopnea index (/h) | 26.6 ± 15.4 | 25.3 ± 18.1 | 22.0 ± 13.7 | NS |
| Central apnea index (/h) | 11.4 ± 11.7 | 9.3 ± 12.3 | 11.2 ± 12.0 | NS |
| Obstructive apnea index (/h) | 0.8 ± 1.2 | 0.5 ± 0.7 | 1.0 ± 1.7 | NS |
| Mixed apnea index (/h) | 1.5 ± 6.1 | 2.5 ± 8.5 | 0.2 ± 0.3 | NS |
| Hypopnea index (/h) | 12.6 ± 8.2 | 13.1 ± 10.8 | 9.6 ± 5.0 | NS |
| Minimum O2 saturation (%) | 84 ± 6 | 87 ± 5 | 84 ± 8 | NS |
| Moderate-to-severe SDB (AHI≥15/h, %) | 19 (63%) | 9 (60%) | 6 (55%) | NS |
- a Data are presented as mean ± SD.
- b ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin β receptor blockers; BSA, body surface area; CRT-D, cardiac resynchronization therapy with defibrillation, ICD, implantable cardioverter–defibrillator; SDB = sleep-disordered breathing.
Acute impact of adaptive servo-ventilation therapy
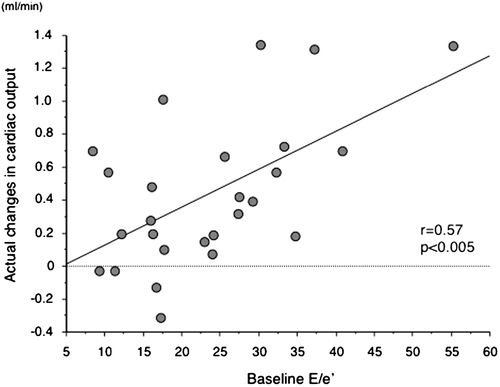
Heart rate and blood pressure were significantly decreased 30 min after the initiation of ASV therapy. No significant differences in LVEDV, maximum LA volume, MR vena contracta width, or LV diastolic function parameters were noted 30 min after the initiation of ASV therapy. However, LVESV was significantly decreased after 30 min of ASV therapy, resulting in an improvement in LVEF. Stroke volume increased significantly in conjunction with a reduction in SVR and an improvement of SAC. Cardiac output was also significantly increased (Table 2). A significant correlation was noted between the absolute increase in stroke volume or cardiac output and the absolute decrease in SVR (r = 0.46, r = 0.63, P < 0.01, respectively). Furthermore, there was a significant correlation between baseline E/e′ and actual change in cardiac output during acute ASV therapy (Figure 2). Using multivariate linear regression analysis, baseline E/e′ was found to be the only independent predictor of the absolute change in cardiac output with acute ASV therapy (t = 2.492, P= 0.0074).
| Baseline | ASV 30 min | P value | |
|---|---|---|---|
| Heart rate (bpm) | 72 ± 14 | 69 ± 13 | <0.0001 |
| Systolic blood pressure (mmHg) | 124 ± 21 | 111 ± 20 | <0.0001 |
| Diastolic blood pressure (mmHg) | 74 ± 14 | 68 ± 14 | <0.005 |
| Stroke volume (mL) | 45 ± 15 | 54 ± 18 | <0.0001 |
| Cardiac output (L/min) | 3.1 ± 1.0 | 3.6 ± 1.1 | <0.0001 |
| LV/LA volumes | |||
| LV end-diastolic volume (mL) | 150 ± 56 | 149 ± 55 | NS |
| LV end-systolic volume (mL) | 102 ± 52 | 96 ± 52 | <0.0001 |
| LV ejection fraction (%) | 34 ± 11 | 39 ± 13 | <0.0001 |
| Maximum LA volume (mL) | 105 ± 46 | 100 ± 45 | NS |
| Minimum LA volume (mL) | 66 ± 45 | 68 ± 44 | NS |
| LV diastolic function | |||
| E wave (cm/s) | 91 ± 37 | 89 ± 38 | NS |
| A wave (cm/s) | 62 ± 37 | 61 ± 34 | NS |
| e′ (cm/s) | 4.2 ± 1.4 | 4.2 ± 1.4 | NS |
| a′ (cm/s) | 4.7 ± 2.4 | 4.5 ± 2.6 | NS |
| E/e′ | 23.3 ± 10.8 | 22.8 ± 10.1 | NS |
| Mitral regurgitation vena contracta width (mm) | 3.3 ± 1.5 | 3.2 ± 1.4 | NS |
| Systemic arterial compliance (mL/mmHg) | 0.96 ± 0.36 | 1.29 ± 0.51 | <0.0001 |
| Systemic vascular resistance (dyne/s/cm5) | 2539 ± 860 | 2026 ± 702 | <0.0001 |
- a Data are presented as mean ± SD.
- b A wave, late diastolic peak flow velocity; a′, mitral annular late diastolic velocity, E wave, early diastolic peak flow velocity; e′, mitral annular early diastolic velocity.
Chronic impact of adaptive servo-ventilation therapy
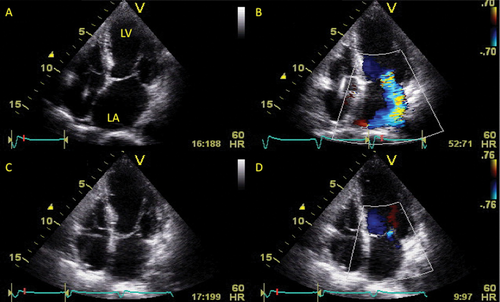
No significant differences in heart rate, blood pressure, or LV inflow velocities were observed in either group between baseline and the end of the follow-up period. In patients undergoing ASV therapy, stroke volume, and cardiac output were increased significantly in conjunction with a reduction in SVR at the end of the follow-up period. Moreover, a significant increase in s′ was noted, a finding which suggests that ASV therapy improved LV longitudinal systolic function. Left ventricular diastolic function parameters were also improved during the follow-up period. In addition to the improvements in LVEF, a significant reduction in LV and LA volumes was also observed at the end of the follow-up period (Table 3). A significant reduction in MR vena contracta width was noted, and there was a good correlation between the reduction in MR vena contracta width and the absolute reduction of LVEDV, LVESV, and maximum LA volume (r = 0.79, r = 0.71, and r = 0.73, P < 0.01, respectively). These beneficial effects were not observed in patients in the withdrawal group. The percentage reduction in LV volumes and maximum LA volume were significantly larger in the ASV group compared with the withdrawal group (LVEDV: 24 ± 11 vs. 10 ± 12%, P< 0.01; LVESV: 38 ± 16 vs. 15 ± 17%, P< 0.005; maximum LA volume: 28 ± 22 vs. 6 ± 27%, P< 0.05). Multivariate linear regression analysis revealed that ASV usage was the only independent predictor for the percent change in LV volumes (LVEDV: t = 3.592, P= 0.0024; LVESV: t = 3.677, P= 0.0020) and that in maximum LA volume (t = 2.205, P= 0.0393) during the follow-up period. A representative case is shown in Figure 3.
| ASV group | Withdrawal group | |||||
|---|---|---|---|---|---|---|
| n = 15 | n = 11 | |||||
| Baseline | Follow-up | P value | Baseline | Follow-up | P value | |
| NYHA class | 2.4 ± 0.5 | 1.5 ± 0.5 | <0.01 | 2.3 ± 0.5 | 2.0 ± 0.8 | NS |
| Heart rate (bpm) | 74 ± 16 | 70 ± 13 | NS | 70 ± 12 | 67 ± 13 | NS |
| Systolic BP (mmHg) | 117 ± 22 | 123 ± 31 | NS | 128 ± 16 | 121 ± 14 | NS |
| Diastolic BP (mmHg) | 72 ± 16 | 66 ± 15 | NS | 75 ± 11 | 67 ± 13 | NS |
| Stroke volume (mL) | 43 ± 15 | 56 ± 16 | 0.0010 | 49 ± 15 | 54 ± 12 | NS |
| Cardiac output (L/min) | 3.13 ± 1.06 | 3.83 ± 0.85 | 0.0037 | 3.35 ± 0.88 | 3.52 ± 0.65 | NS |
| SAC (mL/m2/mmHg) | 0.63 ± 0.22 | 0.69 ± 0.26 | NS | 0.63 ± 0.20 | 0.65 ± 0.11 | NS |
| SVR (dyne/s/cm5) | 2483 ± 965 | 1837 ± 490 | 0.0005 | 2338 ± 600 | 1991 ± 477 | NS |
| E wave (cm/s) | 90 ± 35 | 81 ± 42 | NS | 105 ± 46 | 90 ± 40 | NS |
| A wave (cm/s) | 64 ± 43 | 63 ± 45 | NS | 75 ± 41 | 68 ± 49 | NS |
| e′ (cm/s) | 3.8 ± 1.5 | 5.3 ± 1.5 | 0.0013 | 4.0 ± 1.2 | 4.0 ± 1.5 | NS |
| a′ (cm/s) | 4.2 ± 1.7 | 5.1 ± 2.8 | 0.0266 | 5.6 ± 2.0 | 5.7 ± 4.0 | NS |
| s′ (cm/s) | 3.7 ± 1.1 | 5.3 ± 1.9 | 0.0218 | 4.6 ± 2.0 | 4.4 ± 1.7 | NS |
| E/e′ | 27.1 ± 16.1 | 16.3 ± 8.7 | 0.0075 | 27.2 ± 10.0 | 25.8 ± 16.6 | NS |
| LVEDV (mL) | 169 ± 66 | 129 ± 61 | <0.0001 | 171 ± 60 | 158 ± 73 | NS |
| LVESV (mL) | 122 ± 63 | 79 ± 60 | <0.0001 | 117 ± 58 | 106 ± 71 | NS |
| LVEF (%) | 30 ± 11 | 43 ± 14 | 0.0001 | 33 ± 11 | 38 ± 13 | NS |
| Max LAV (mL) | 113 ± 54 | 85 ± 61 | 0.0006 | 100 ± 36 | 95 ± 50 | NS |
| Min LAV (mL) | 70 ± 56 | 50 ± 49 | 0.0111 | 61 ± 31 | 60 ± 46 | NS |
| MR VCW (mm) | 3.5 ± 1.3 | 1.6 ± 2.4 | 0.0005 | 3.1 ± 2.4 | 3.0 ± 2.7 | NS |
- a Data are presented as mean ± SD or numbers (%).
- b BP, blood pressure; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; LV, left ventricle; Max LAV, maximum left atrial volume; Min LAV, minimal left atrial volume; MR VCW, mitral regurgitation vena contracta width; NYHA, New York Heart Association; s′, mitral annular peak systolic velocity; SAC, systemic arterial compliance; SVR, systemic vascular resistance.
Observer variabilities
Intra-observer variabilities for measuring LVEDV, LVESV, and maximum LA volume were 8, 8, and 5%, respectively. Corresponding inter-observer variabilities were 10, 10, and 8%, respectively.
Discussion
The major findings in this study were as follows: (i) acute ASV therapy produced a significant increase in stroke volume and cardiac output in conjunction with a reduction of SVR, 30 min after the initiation of ASV therapy, (ii) a significant correlation between baseline E/e′ and the absolute increase in cardiac output was noted, and baseline E/e′ was an independent predictor of the acute increase in cardiac output, (iii) chronic ASV therapy produced a significant reduction of LV and LA volumes, resulting in an improvement in LV systolic and diastolic function, (iv) a significant reduction of functional MR was noted during chronic ASV therapy, and there were good correlations between the reduction of MR severity and absolute reduction of LVEDV, LVESV, and LA volume, and (v) Multivariate regression analysis showed that chronic ASV therapy was an independent predictor for both LV and LA reverse remodelling.
Acute effects of adaptive servo-ventilation therapy on cardiac function
We have demonstrated that acute ASV therapy significantly increases both stroke volume and cardiac output that is associated with an improvement of SVR and SAC as parameters of afterload. In addition, these beneficial effects of ASV therapy were more remarkable in patients who had elevated baseline E/e′, a finding which is in agreement with previous acute CPAP studies.16,17 Adaptive servo-ventilation reduces LV afterload by increasing intrathoracic pressure and reducing LV transmural pressure. Pinsky et al.18 suggested that cardiac output may increase in patients with CHF, because the positive effects of decreasing afterload outweigh the negative effects of decreasing preload using PAP therapy. The significant reduction in heart rate was also in agreement with previous acute CPAP studies.16,19 This suggests that ASV may either reduce cardiac sympathetic nerve activity or increase parasympathetic nerve activity by stimulating pulmonary stretch receptors due to lung inflations.20
Chronic effects of adaptive servo-ventilation therapy on cardiac function
Previous studies have reported that chronic ASV therapy significantly improves nocturnal abnormal breathing patterns and sleep quality compared with other PAP devices in CHF patients with CSR.21 In addition, several studies have reported that chronic ASV therapy not only eliminates SDB but also increases LVEF.22–24 Left ventricular ejection fraction is a standard parameter for the assessment of global LV systolic function. However, a normal LVEF does not always guarantee normal LV systolic function. Functional MR is accompanied frequent comorbidity in patients with heart failure and contributes to disease progression.25 Forward stroke volume might be decreased in patients with normal LVEF and significant functional MR.26 Therefore, it is important to evaluate haemodynamics, forward stroke volume, and the severity of functional MR for the assessment of cardiac function in patients with CHF. We observed that chronic ASV therapy produces an increase in LVEF and forward SV with a significant reduction of left chamber volumes and functional MR. The reductions in LV and LA volumes in the current study were comparable to those achieved by ß-blocker therapy or cardiac resynchronization therapy.27,28 Potential mechanisms for this reverse remodelling might be the improvement of cardiac contractility and the reduction of SVR.29 A significant reduction of MR vena contracta width was noted only in patients undergoing chronic ASV therapy, and there was a good correlation between the reduction of MR vena contracta width and of the reduction in LV and LA volumes. Tkacova et al.30 previously reported that 3 months of CPAP therapy induced a significant reduction of MR severity and improvement of LVEF, assessed using radionuclide angiography. Our results suggest that left chamber reverse remodelling might at least partly contribute to the reduction in functional MR.
Bitter et al.31 demonstrated that ASV therapy increases e′ and decreases E/e′ in patients with heart failure with normal LVEF and CSR. We also demonstrated that chronic ASV therapy is accompanied by a significant increase in s′, e′, and a′, as well as a decrease in E/e′ even in CHF patients with decreased LVEF. Chronic ASV therapy may produce LV and LA reverse remodelling resulting in an improvement of both LV systolic and diastolic function.
Study limitations
Several limitations of this study should be addressed. The application of ASV therapy was different between the acute and chronic studies. The acute study was performed during the daytime, whereas chronic ASV therapy was performed when the patient was asleep. If patients are awake without apnoea, ASV theoretically provides immovable same pressure support. However, the averaged inspiratory pressure support during the acute study was 4.2 ± 0.8 cmH2O, and thus it was not minimal pressure support (3 cmH2O). This finding suggests that some patients fell asleep or had respiratory instability even during acute ASV study. Although baseline left chamber volumes and function were similar between the ASV group and the withdrawal group in the chronic study, the allocation to ASV therapy was not randomized. Only half of the study patients could tolerate ASV therapy. However, similar rates of compliance have been reported in previous non-randomized studies of ASV.22,23 The study sample size was relatively small. Thus, we cannot extrapolate our findings to the general heart failure population. A multicentre randomized controlled trial to investigate the effects of ASV in CHF patients is now warranted.
Conclusions
The beneficial impact of ASV therapy in patients with CHF was different in the acute and chronic phases. Acute ASV therapy produced favourable effects on haemodynamics and stroke volume. In contrast, chronic ASV therapy produced left chamber reverse remodelling and a reduction of MR.
Acknowledgements
We are grateful to Teijin Pharma Ltd. for their continued support. We also acknowledge Yoshitaka Fujita for his continuous home care support of patients undergoing chronic ASV.
Conflict of interest: none declared.




