Occurrence of late gadolinium enhancement is associated with increased left ventricular wall stress and mass in patients with non-ischaemic dilated cardiomyopathy
Abstract
Aims
Occurrence of late gadolinium enhancement (LGE) as assessed by cardiac magnetic resonance (CMR) imaging has been attributed to various myocardial injuries. We hypothesized that LGE is associated with left ventricular (LV) wall stress.
Methods and results
We examined 300 patients with suspected non-ischaemic dilated cardiomyopathy. Cardiac magnetic resonance was used to assess LV volume, mass, wall stress, and LGE. Increased LV end-diastolic wall stress (> 4 kPa) was found in 112 patients (37 %), and increased end-systolic wall stress (>18 kPa) in 121 patients (40%). Presence of LGE was observed in 93 patients (31%). End-diastolic (94 ± 43 vs. 79 ± 42 ml/m², P = 0.006) and end-systolic LV volumes (62 ± 44 vs. 44 ± 37 ml/m², P < 0.001) and LV mass (95 ± 34 vs. 78 ± 31 g/m², P < 0.001) were increased in patients exhibiting LGE. In particular, LV end-diastolic and end-systolic wall stress were increased (4.5 ± 2.8 vs. 3.6 ± 3.0 kPa, P = 0.025; 19.6 ± 9.1 vs. 17.5 ± 8.2 kPa, P = 0.045). Late gadolinium enhancement was observed more frequently than would be expected from random occurrence in patients with increased end-diastolic (39 vs. 26%, P = 0.020) and end-systolic wall stress (41 vs. 24%, P = 0.002). Both normal end-diastolic and end-systolic wall stress had a high negative predictive value for LGE (75 and 76%).
Conclusions
The present study shows that occurrence of LGE in cardiomyopathy is associated with increased LV wall stress and mass. Suspected causes are an increased capillary leakage by stretch, impaired contrast agent redistribution, or increased diffusion distances. It is proposed that LGE should be considered as a potential prognostic determinant of heart failure and severe arrhythmias.
See page 927 for the editorial comment on this article (doi:10.1093/eurjhf/hfr084)
Introduction
Late gadolinium enhancement (LGE) as assessed using cardiac magnetic resonance (CMR) imaging, is associated with poor prognosis in various heart diseases. Occurrence of LGE is caused by prolonged interstitial contrast agent distribution that shortens repolarization during CMR and can thus be used for image contrast enhancement.1 Gadolinium-chelated compounds are used as paramagnetic contrast agents. Due to their small molecular weight, they can leave the vasculature and access the interstitial space. Previously, the occurrence of LGE has been linked to myocardial fibrosis.2,3 Late gadolinium enhancement has been found in infarcted areas in ischaemic heart disease due to fibrotic remodelling.4–7 Its role in non-ischaemic cardiomyopathy remains controversial.8–10 Various myocardial injuries such as inflammation and storage diseases are thought to be linked to LGE.11 Of note, the occurrence of LGE was associated with a poor prognosis in patients with non-ischaemic dilated cardiomyopathy.12,13
While some of the prognostic values of LGE can be attributed to fibrosis,14 other as yet unexplored influences are also likely. An increase in ventricular wall stress could be of particular importance in this respect. Using CMR, wall stress can be determined accurately based on ventricular volume, mass, and intraventricular pressure. We hypothesized therefore, that increased ventricular wall stress is associated with the occurrence of LGE and may explain the functional and prognostic significance of LGE.
Methods
A total of 300 patients who were admitted to our heart centre with suspected non-ischaemic cardiomyopathy were examined. Since the patients exhibited a wide range of LV function and volumes, the broad spectrum from severe systolic impairment to preserved function as well as from severe dilatation to non-dilatation could be examined.
All patients suffered from exertional or non-exertional chest pain or dyspnoea, exhibited 12-lead standard electrocardiogram (ECG) alterations or positive exercise testing. Heart valve diseases were excluded. The presence of coronary artery disease was excluded by heart catheterization before entry into the study as part of routine diagnostics. Thereby, left ventricular end-diastolic pressure (LVEDP) and left ventricular end-systolic pressure (LVESP) and aortic pressure were assessed using Statham pressure transducers (standardized method). When indicated, patients received concomitant medication for therapy of heart failure. Cardiac magnetic resonance was performed following a standardized protocol to evaluate the suspected cardiomyopathy.15,16 The study was approved by the local institutional ethics committee, and written consent was obtained from all study participants.
Assessment of left ventricular volume, mass, late gadolinium enhancement, and left ventricular wall stress
All patients underwent CMR (1.5 T, Sonata, Siemens Medical Systems, Erlangen, Germany). To assess LV volumes (LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume), function (LVEF, left ventricular ejection fraction) and myocardial mass, a stack of short-axis views from base to apex was obtained using ECG-gated steady-state free precession sequences (TrueFISP, slice 6 to 8 mm).17 The occurrence of LGE was assessed 10 to 12 min after contrast agent application (0.2 mmol gadolinium/kg of body weight) using fast low-angle shot sequences (TurboFLASH).15 All examinations were performed in a double-blind manner. The investigators reading the CMR images (M.B.R. and J.H.F.) were not aware of pressure or wall stress data. Investigators calculating LV wall stress (P.A. and H.R.) were unaware of LGE findings.
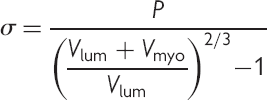 (1)
(1)Patient stratification
Patients were stratified in accordance with the presence or absence of LGE. Furthermore, patients were divided in quartiles of several functional parameters (LVEDV, LVESV, LVEF, LV mass, LV end-diastolic wall stress, and LV end-systolic wall stress).
Statistical analysis
Fisher's exact test was used for comparison of categorical variables between 2 groups, and χ2 test for >2 groups. Between-group comparisons of means were made by Student's t-test. When the normality test failed, a Mann–Whitney rank sum test was performed. Comparisons between >2 groups were made by one-way analysis of variance. Tukey post hoc test was used for multiple comparisons. When the normality test failed, Kruskal–Wallis one-way analysis of variance on ranks and, if appropriate, Dunn's post hoc test were performed for pair-wise comparisons. Holm−Sidak's method was used for multiple comparisons of the frequency of LGE of eight groups. Values are expressed as means ± standard deviation. Statistical significance was assumed as P < 0.05.
Results
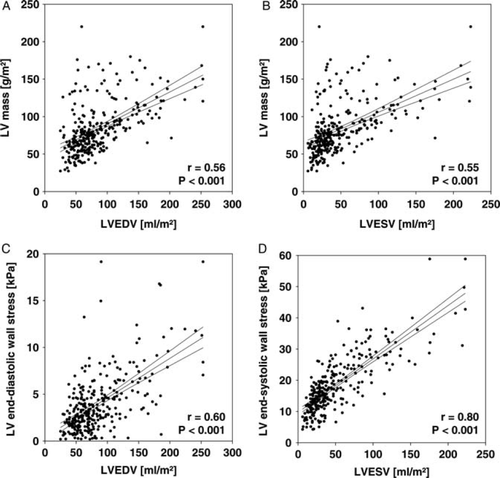
The 300 patients comprised a wide spectrum of cardiac function ranging from severe systolic impairment (LVEF of 7%) to normal systolic function with a mean LVEF of 47 ± 17%. Patient characteristics are summarized in Table 1. Left ventricular dilatation (LVEDV ≥90 mL/m²) was present in 96 patients (32%); and reduced ventricular systolic function (LVEF ≤50%) was observed in 159 patients (53%). Left ventricular mass, indicating the extent of hypertrophy, was correlated with LV end-diastolic and end-systolic volumes. However, LV end-diastolic wall stress and LV end-systolic wall stress was also correlated with end-diastolic and end-systolic volume (Figure 1). An increased LV end-diastolic wall stress (>4 kPa) was found in 112 patients (37%) and an increased LV end-systolic wall stress (>18 kPa) in 121 patients (40%).
| Total | No LGE | LGE | P | |
|---|---|---|---|---|
| n = 300 | n = 207 | n = 93 | ||
| Age (years) | 50 ± 15 | 50 ± 15 | 51 ± 16 | ns |
| Male | 209 (70%) | 138 (67%) | 71 (76%) | ns |
| Body mass index (kg/m²) | 27 ± 4 | 27 ± 4 | 26 ± 4 | ns |
| Body surface area (m²) | 2,0 ± 0.2 | 2,0 ± 0.2 | 2,0 ± 0.2 | ns |
| Medication | ||||
| β-Blockers | 202 (67%) | 137 (66%) | 65 (70%) | ns |
| Diuretics | 169 (56%) | 105 (51%) | 64 (93%) | 0.014 |
| ACE inhibitors/AT antagonists | 184 (61%) | 117 (57%) | 67 (72%) | 0.015 |
| Aldosterone antagonists | 80 (27%) | 48 (23%) | 32 (34%) | ns |
| Aortic pressure | ||||
| Systolic (mm Hg) | 127 ± 24 | 129 ± 24 | 123 ± 23 | ns |
| Diastolic (mm Hg) | 69 ± 13 | 69 ± 13 | 68 ± 14 | ns |
| Mean (mm Hg) | 91 ± 15 | 92 ± 15 | 90 ± 16 | ns |
| Left ventricular pressure | ||||
| LVEDP (mm Hg) | 16 ± 10 | 15 ± 10 | 18 ± 10 | 0.007 |
| LVESP (mm Hg) | 130 ± 24 | 132 ± 23 | 125 ± 23 | 0.016 |
| Left ventricular volume, function, and mass | ||||
| LVEDV (mL/m²) | 84 ± 43 | 79 ± 42 | 94 ± 43 | 0.006 |
| LVESV (mL/m²) | 49 ± 40 | 44 ± 37 | 62 ± 44 | <0.001 |
| LVEF (%) | 47 ± 17 | 50 ± 17 | 40 ± 17 | <0.001 |
| LV mass (g/m²) | 83 ± 33 | 78 ± 31 | 95 ± 34 | <0.001 |
| Left ventricular wall stress | ||||
| End-diastolic (kPa) | 3.9 ± 3.0 | 3.6 ± 3.0 | 4.5 ± 2.8 | 0.025 |
| End-systolic (kPa) | 18.2 ± 8.5 | 17.5 ± 8.2 | 19.6 ± 9.1 | 0.045 |
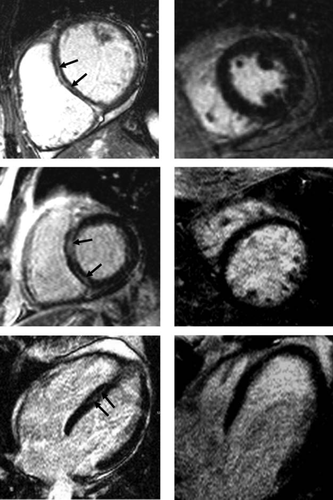
Presence of LGE was observed in 93 (31%) patients. Examples of LGE in short- and long-axis views as assessed using CMR are shown in Figure 2. Typically, but not always, LGE appears as streaks within the interventricular septum. Examples of patients without LGE are also given.
A multiple regression analysis including LVEDP, LVESP, LVEDV, LVESV, and LV mass as independent variables and LGE as dependent, revealed influences of LV mass (P = 0.0028), LVESV (P = 0.0291), and LVEDV (P = 0.0444), which are integral components of end-diastolic and end-systolic LV wall stress. No significant influences of LVEDP and LVESP on the occurrence of LGE were found.
Stratification of patients according to the presence or absence of LGE showed that impaired heart function was associated with the occurrence of LGE (Table 1). In patients with LGE, LVEF and LV end-systolic pressure were decreased, whereas LV end-diastolic pressure was increased. Furthermore, end-diastolic and end-systolic LV volumes and LV mass were increased. It was interesting that LV end-diastolic and end-systolic wall stress were also increased. Late gadolinium enhancement was observed more frequently than would be expected from random occurrence in patients with increased end-diastolic (39 vs. 26%, P = 0.020) and end-systolic wall stress (41 vs. 24%, P = 0.002, Fisher's exact test) when compared with normal wall stress. Using >4 kPa as the cut-off value for increased end-diastolic wall stress, ROC-analysis revealed a sensitivity of 47% and a specificity of 70% to predict the occurrence of LGE [area under the curve (AUC) 0.611]. Normal end-diastolic wall stress was associated with a negative predictive value (i.e. absence of LGE) of 75%. A cut-off value of >18 kPa for increased end-systolic wall stress had a sensitivity of 54% and a specificity of 67% to predict the occurrence of LGE (AUC 0.574). The negative predictive value was 76%.
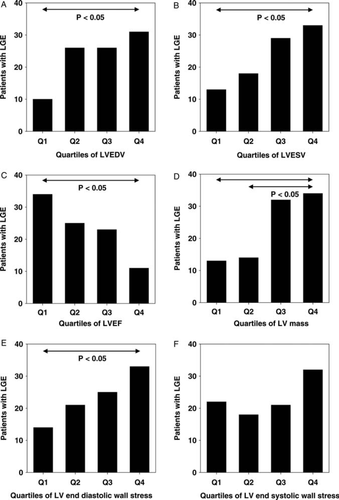
Influences on LGE were further examined by using quartiles of LV end-diastolic and end-systolic volumes, LVEF, LV mass, as well as LV end-diastolic wall stress and LV end-systolic wall stress (Figure 3). The frequency of LGE was associated with increased LV end-diastolic and end-systolic volume, decreased LVEF, and increased LV mass, respectively. Of particular interest, LGE occurred more frequently in patients with increased end-diastolic and end-systolic wall stress.
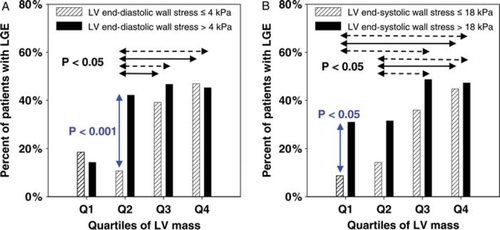
To reveal potential interdependent influences of LV mass and wall stress on the occurrence of LGE, further subgroup analysis was performed. First, patients were divided into quartiles of LV mass. Then patients with a given range of mass within each quartile were divided according to normal or increased end-diastolic and end-systolic wall stress (Figure 4). The occurrence of LGE was associated with increased LV mass. However, LGE occurred more frequently in patients with increased end-diastolic wall stress compared with normal wall stress within quartiles 2 and 3 (by trend) of LV mass and, of note, in patients with increased end-systolic wall stress within quartile 1, and in quartiles 2 and 3 (by trend). Thus, an increased wall stress is associated with LGE which, however, also arises from marked LV hypertrophy.
Discussion
The present study shows that increased ventricular wall stress and mass are associated with the occurrence of LGE in patients with non-ischaemic dilated cardiomyopathy. Wall stress is an important determinant of heart failure and thus may explain part of the unfavourable prognostic role of LGE.19,20 Assessment of LGE is based on CMR image acquisition after a certain delay subsequent to contrast agent application. Therefore, prolonged contrast agent deposition is required. Since usual gadolinium-based compounds are of low molecular weight, movement from the vasculature into the interstitial space occurs. Increased extravasal transfer, impaired redistribution into the vasculature, and an increase in the interstitial space per se can prolong tissue clearance of the contrast agent.
Wall stress is mainly determined by ventricular volume, myocardial mass, and pressure.21 An increase in volume, i.e. ventricular dilatation, is associated with myocardial hypertrophy. Therefore, both LV mass and wall stress are correlated with LV volume. Beyond a certain degree of dilatation, the increase in mass is not sufficient to prevent a rise in wall stress. Since a given interstitial space within the myocardial wall can obviously not sense the inner ventricular volume, one cannot assume that an increase in LV volume per se leads to myocardial LGE. It appears that the volume contributes to wall stress and that at a cellular level increased stretch facilitates LGE. As regards potential mechanisms, it has been suggested that increased ventricular wall stress enhances capillary leakage and thus favours emission of contrast medium from the vasculature into the interstitial space.22 Also, redistribution into the vasculature can be impaired, which prolongs its venous clearance. Thus, there is a net increase in the tissue content of contrast medium.23 Conversely, normal wall stress has a favourable negative predictive value for LGE. Thus, increased wall stress seems to be a mandatory, but not an obligatory condition for the occurrence of LGE. In addition, LV mass including myocyte hypertrophy and expansion of the interstitial space is suspected to be directly associated with LGE, which may be associated with the model of increased diffusion distances.9,24–27 Of note, increased wall stress precedes the development of LV hypertrophy.28 Since increased wall stress was also associated with LGE when LV mass was not markedly increased, it can be deduced that both wall stress and LV hypertrophy can cause LGE independently of each other.
Several cardiac entities are associated with the occurrence of LGE. The latter is a typical finding in patients after myocardial infarction when the ischaemic area has undergone structural and functional remodelling leading to marked wall thinning and fibrosis. The feasibility of quantifying the infarct area and deducing potential prognostic implications has been demonstrated several times.2,4,5,29,30 Cardiac magnetic resonance is used for the differentiation of heart failure related to coronary artery disease and dilated cardiomyopathy.31 However, the interpretation of LGE in patients with cardiomyopathy remains controversial.10 Myocardial remodelling of patients with ventricular dilatation is less well described, but frequently affects several remote myocardial areas or the complete LV. From histopathological studies it is known that myocardial remodelling can be associated with a marked increase of the collagen volume fraction in patients with hypertensive (7.6 ± 0.7 vs. 0.5 ± 0.2%, P < 0.01) and dilated heart disease.32 It has been shown in necropsy studies on transmural left ventricular sections of dilated cardiomyopathy that the extent of fibrosis increases from epicardium to endocardium, and from the right to the left side of the septum.33 In cardiomyopathy, LGE typically occurs as mid-wall streaks, and is patchy or diffuse.31 Of note, no differences in the collagen volume fraction were found in patients with or without LGE.34 Other putative factors also associated with LGE should be considered,35 such as (transient) myocarditis36 and amyloid deposition.37 Also in this respect it seems unlikely that fibrosis is the primary determinant of LGE in dilated cardiomyopathy.38 However, the interpretation of LGE in patients with cardiomyopathy remains controversial.10
Late gadolinium enhancement is associated with a poor prognosis.12,13 However, in a large study of 140 patients with dilated cardiomyopathy, the prognostic value of LGE for survival was only found by univariate, but not by multivariate analysis. This supports the hypothesis the LGE per se is not an independent prognostic predictor but rather a dependent surrogate marker.39 It has previously been shown by positron emission tomography that the regional myocardial blood flow reserve is impaired in patients with non-ischaemic dilated cardiomyopathy, which was associated with increased echocardiography-based systolic wall stress.40 Therefore, increased ventricular wall stress should be considered as a potential causative determinant of LGE by affecting myocardial blood flow and contrast agent distribution and redistribution in an altered interstitial space. Increased wall stress may thus also be the underlying mechanism of a worse prognosis20 which has previously been linked to LGE. Although the causes of the onset of dilatation remain ill defined in the clinical setting, it appears that an increased wall stress contributes to the progression of dilatation. Since LGE was associated with an increased wall stress, it can be deduced that LGE is a marker of a causative factor contributing to dilatation.
Limitations
It could be argued that LGE is patchy and not homogenously distributed in cardiomyopathy. The thick-walled sphere model used allows calculation of mean wall stress assuming ideal and homogenous conditions of the whole LV. It can be assumed that local variances of wall stress, which could not be detected using the present spherical model, have additional influences and should not be ignored. Globally increased wall stress can promote net contrast distribution into the interstitial space and potentially additive local influences can contribute to rendering the LGE detectable. This idea is supported by the present finding that normal wall stress has a favourable predictive value for the absence of LGE. Similar mechanisms can also contribute to the occurrence of LGE in the border zone of infarcted areas, where a marked increase in local wall stress is known to occur. In addition, increased intraventricular pressure can exceed the peripheral coronary perfusion and lead to myocardial ischaemia particularly in the middle of the triple-layered myocardium affecting the sensitive circular fibres first, which could contribute to the findings, e.g. by impaired contrast agent clearance.12,31,40 In the present study, the majority of patients were receiving chronic heart failure therapy. It can be assumed that ventricular wall stress is higher in patients with acute decompensated heart failure without adequate afterload reduction, which may also explain the temporary occurrence and reversibility of LGE in some patients with cardiomyopathy.
Future directions
In the present study, the occurrence of LGE was shown to be associated with LV wall stress and mass. Ventricular wall stress is an important functional parameter in heart failure. Left ventricular mass is known to be an independent prognostic predictor.41 It is known from experimental animal studies that increased myocardial stretch and neurohumoral adaptations associated with ventricular dilatation predispose to ventricular arrhythmias,42–46 e.g. mediated by stretch-activated ion channels. Accordingly, it can be assumed that increased LV wall stress increases arrhythmia risk, whereby regional wall stress can contribute to local inhomogeneities.47 In particular, the occurrence of sudden cardiac death due to severe ventricular arrhythmias could be linked to wall stress-associated LGE. Work is in progress to evaluate LGE as a potential prognostic determinant for the progression of heart failure, occurrence of sudden cardiac death, and its usefulness in risk stratification for prophylactic treatment with an implanted cardioverter–defibrillator.
Conclusions
The occurrence of LGE was associated with increased LV wall stress and mass. Based on the present findings, increased ventricular wall stress appears to be a previously unrecognized major determinant of LGE. It is proposed that evaluation of LGE should be considered as a potential prognostic determinant of heart failure and severe arrhythmias.
Funding
The study was supported by a Research Grant of the University Medical Center Giessen and Marburg.
Conflict of interest: none declared.




