Relationship between peak cardiac pumping capability and selected exercise-derived prognostic indicators in patients treated with left ventricular assist devices
Abstract
Aim
Exercise-derived variables have been used in the assessment of functional capacity and prognosis in patients with chronic heart failure. The aim of this study was to assess the relationship between cardiac pumping capability represented by peak cardiac power output and peak oxygen consumption, anaerobic threshold, ventilatory efficiency slope, and peak circulatory power in patients undergoing the ‘Harefield Protocol’.
Methods and results
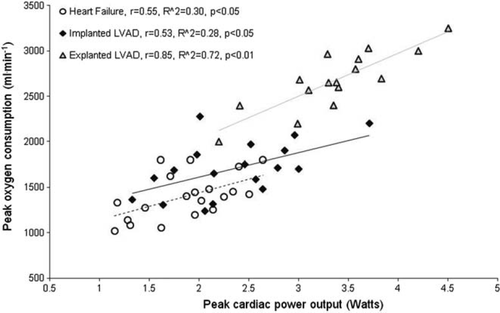
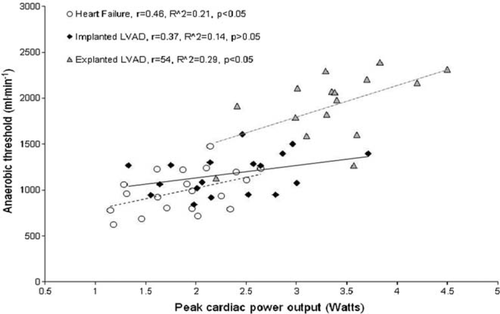
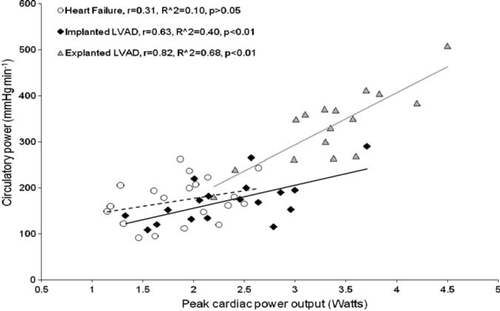
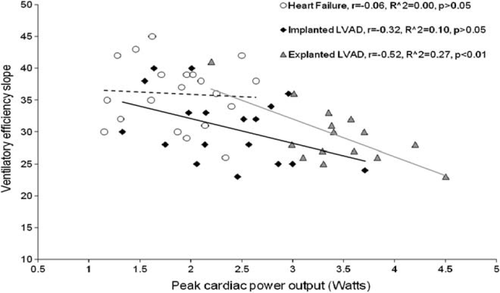
Haemodynamic and gas exchange measurements were undertaken during a graded treadmill exercise test. They were performed on 54 patients—18 implanted with left ventricular assist devices (LVADs), 16 explanted (recovered), and 20 moderate-to-severe heart failure patients. Peak oxygen consumption was only highly correlated with peak cardiac power output in explanted LVAD (r = 0.85, P< 0.01), but not in implanted LVAD and heart failure patients (r = 0.55 and 0.53, P< 0.05). The anaerobic threshold was only modestly correlated with peak cardiac power output in heart failure and explanted (r = 0.46 and 0.54, P< 0.05) and weakly in implanted LVAD patients (r = 0.37, P< 0.05). Peak cardiac power output was well correlated with peak circulatory power in LVAD explanted and implanted (r = 0.82, P< 0.01; r = 0.63, P< 0.01) but not in heart failure patients (r = 0.31, P> 0.05). Ventilatory efficiency slope was only moderately correlated with peak cardiac power output in LVAD-explanted patients (r = −0.52, P< 0.05).
Conclusion
Exercise-derived prognostic indicators demonstrate limited capacity in reflecting cardiac pumping capability in patients treated with LVADs and should therefore be used with caution in interpretation of cardiac organ function.
Introduction
Left ventricular assist devices (LVADs) have been used as an effective therapeutic option in patients with advanced heart failure either as a bridge to transplantation, as destination therapy, or as a bridge to recovery.1,2 A strategy of combined mechanical (i.e. LVAD) and pharmacological therapy, the so-called ‘Harefield Protocol’, has been proposed with the β2-adrenoreceptor agonist clenbuterol used to stimulate physiological hypertrophy during LVAD support.3,4
Cardiopulmonary exercise testing data have been widely used in chronic heart failure patients to assess functional capacity and to determine prognosis.5–7 Specifically, in patients implanted with LVADs cardiopulmonary exercise testing has been used to assess physical functional capacity and to identify those patients in whom the device can be explanted.3,4,8 Conventionally measured exercise-derived variables, used in functional and treatment assessment, have also been shown to be powerful predictors of prognosis in heart failure patients. These include peak oxygen consumption,9,10 anaerobic threshold,11 ventilatory efficiency slope,7,12,13 and peak circulatory power.14,15
Cardiac power output is a unique central haemodynamic measure that demonstrates overall cardiac function and is the product of simultaneously measured cardiac output and mean arterial pressure.16 By incorporating both pressure and flow domains of the cardiovascular system, cardiac power output is a unique, direct, and integrative measure of cardiac pumping capability.17 In addition to flow, the heart needs to generate pressure—without which blood in the circulation would come to a standstill, due to the forces opposing flow, the frictional and separational forces. According to the Law of Conservation of Energy, to maintain the circulation, the hydraulic energy lost in the vasculature has to be replenished by the energy imparted by the heart.16,17 Therefore, using modern physiological terms, the function of the heart is to produce adequate hydraulic energy to maintain circulation.17 The way to assess the amount of hydraulic energy imparted into the circulation by the heart is by measuring cardiac power output.16,17 Not surprisingly, peak cardiac power output has been shown to be a most powerful predictor of prognosis and mortality in patients with chronic heart failure.18
Recently, we have shown that cardiac power output differentiates well during cardiac restoration using LVADs19 and emphasizes the benefits of this therapy in terms of organ function at rest and peak exercise.20 Whether conventionally reported exercise-derived variables reflect cardiac pumping capability in patients implanted with LVADs and those explanted due to myocardial recovery remains unknown. Therefore, the present study aimed to assess the relationship between peak cardiac function and other exercise-derived variables in patients undergoing the ‘Harefield Protocol’ as previously described.1,3,4 We hypothesize that commonly reported exercise-derived prognostic variables do not strongly correlate with peak cardiac power output.
Methods
Patients
The study population consisted of 54 male patients. Patients attended for cardiopulmonary exercise testing at Harefield Hospital, UK. Exclusion criteria were: inability to perform treadmill exercise tests, inability to exercise beyond anaerobic threshold, symptomatic angina limiting exercise, and unwillingness to provide a consent form. Out of the 54 patients recruited, 20 were patients with moderate-to-severe heart failure, 18 patients were implanted with an LVAD ongoing, and 16 patients had been explanted with LVAD due to myocardial recovery. Patients’ clinical characteristics and medication are presented in Table 1.
| Variable | Heart failure | Implanted | Explanted LVAD | |
|---|---|---|---|---|
| (n = 20) | LVAD (n = 18) | (n = 16) | ||
| Age (years) | 45 ± 10 | 39 ± 14 | 41 ± 14 | |
| Age range (years) | 22–64 | 19–58 | 18–63 | |
| Body mass index (kg m2) | 26.8 ± 4.7 | 25.5 ± 4.1 | 26.7 ± 4.0 | |
| Left ventricular ejection fraction (%) | 32 ± 14* | 50 ± 08 (LVAD on) | 58 ± 14 | |
| Aetiology of heart failure | ||||
| Idiopathic dilated cardiomyopathy | 17 | 18 | 16 | |
| Coronary heart disease | 3 | – | – | |
| Medical therapy | ||||
| ACE inhibitors | 15 (75%) | 13 (72%) | 12 (75%) | |
| Beta-blockers | 17 (85%) | 15 (83%) | 13 (81%) | |
| Diuretics | 18 (90%) | 3 (17%) | 12 (75%) | |
| Angiotensin II antagonists | 7 (35%) | 3 (17%) | 6 (38%) | |
| Aldosterone antagonists | 17 (85%) | 15 (83%) | 9 (56%) | |
| Digoxin | 15 (75%) | 11 (61%) | 13 (81%) | |
| Antiarrhythmic agents | 5 (25%) | 2 (11%) | 6 (38%) | |
| Clenbuterol | – | 4 (22%) | – | |
- a *P< 0.05, heart failure vs. implanted LVAD and explanted LVAD.
- b Reprinted from19 with permission from Elsevier
Fifty-five per cent of the heart failure group had New York Heart Association (NYHA) Class IV, and 45% had NYHA Class III heart failure symptomatology. The pathogenesis of heart failure was ischaemic heart disease in 15%, and dilated cardiomyopathy in 85%. Left ventricular ejection fraction ranged from 15 to 55%.
All LVAD patients were implanted with a continuous blood flow LVAD. Fourteen patients had a HeartMate II (Thoratec Corporation, CA, USA) LVAD and four patients had a HeartWare (HeartWare Limited, Miami, USA) LVAD. All LVAD patients had dilated cardiomyopathy. Left ventricular ejection fraction (measured on pump) ranged from 45 to 68%. Fourteen patients were in the first, and four patients were in the second phase of the specific ‘Harefield Recovery Protocol'4 in which additionally the β2-adrenergic-receptor agonist clenbuterol was administered. The first stage of the ‘Harefield Recovery Protocol’ was intended to enhance reverse remodelling whereas the second stage aimed to induce ‘physiological hypertrophy’ by using clenbuterol. Time since device implantation averaged 219 ± 87 days, with a range of 62–388 days. The indication for insertion of the LVAD was the development of severe heart failure that was not responsive to intensive medical treatment, including inotropic support, with evidence of impeding or actually multiorgan failure due to low cardiac output.4 At the time of data collection in all LVAD patients, the device was set up at the optimal speed rate ranging from 9000 to 9600 r.p.m. for the HeartMate II LVAD and between 2600 and 3500 r.p.m. for the HeartWare LVAD.
Most explanted LVAD patients (87%) were in NYHA Class I and 13% were in NYHA Class II, respectively. All LVAD-explanted patients had dilated cardiomyopathy. Left ventricular ejection fraction of the explanted patients ranged from 50 to 72%. The mean period of LVAD support had been 396 days (range 22–638). Explantation was considered once the following criteria were met while the LVAD was off for 15 min: a left ventricular end-diastolic diameter of <60 mm: a left ventricular end-systolic diameter of <50 mm and an left ventricular ejection fraction (LVEF) of >45%; a left ventricular end-diastolic pressure (or pulmonary-capillary wedge pressure) of <12 mmHg; a resting cardiac index of >2.8 L/min/m2 of body surface area; and maximal oxygen consumption with exercise of >16 mL/kg/min and an increase in minute ventilation relative to the carbon dioxide production (ventilatory response) of <34.4 Patients in the present study were tested at an average of 3.3 ± 1.1 years (range 0.3–5.8) following device explantation. All LVAD-explanted patients completed two stages of pharmacologic regimen described by Birks et al.4
All procedures complied with Declaration of Helsinki and the study was approved by the ethics committee of the Royal Brompton and Harefield Foundation Trust. All patients who participated in the study provided written informed consent.
Testing procedure
On arrival in the transplant exercise laboratory, the patients’ weight and height were measured. The electrocardiogram (ECG) electrodes were attached according to the standard lead configuration for exercise testing and the ECG cables were connected. The patient then sat on a chair and following a 5min resting period, arterial blood pressure was assessed from the brachial artery by cuff sphygmomanometry. This was followed by measurement of cardiac output in a seated position using the inert gas rebreathing methodology (Innocor, Innovision, Odense, Denmark) as previously described.21 Briefly, the Innocor device employs a rebreathing system that uses an oxygen-enriched mixture of an inert soluble gas (0.5% nitrous oxide) and an inert insoluble gas (0.1% sulphur hexafluoride) from a 4-L prefilled anaesthesia bag. Photoacoustic analysers measure gas concentrations over a five-breath interval. Nitrous oxide concentration decreases during the rebreathing manoeuvre at a rate proportional to pulmonary blood flow allowing estimation of cardiac output. Three to four respiratory cycles are needed to obtain a value for nitrous oxide washout.
Following cardiac output measurement at rest, the patient was connected to the Oxycon Pro (Jaeger, Wurzburg, Germany) Metabolic Cart via a disposable pneumotach attached to the face mask. Oxygen consumption, carbon dioxide production, and minute ventilation were measured. After 3min of resting metabolic measurements patient with mild-to-severe heart failure and those implanted with LVAD performed a modified Bruce protocol whereas those patients explanted with LVAD performed the Bruce protocol. Continuous breath-by-breath sampling of respiratory gases and heart rate measurements were undertaken; during the last 30 s of each exercise stage arterial blood pressure was measured. Patients were instructed to give an ∼1-min warning before they felt they would end the exercise so that a final cardiac output rebreathing measurement was obtained at the peak of exercise; this is because the Innocor device takes ∼45 s to boot up followed by 15 s to make the measurement. The patient continued to exercise through the measurement and cool down was started after it was complete. At the same time during the rebreathing manoeuvre, peak arterial blood pressure was measured.
Peak oxygen consumption was defined as the average oxygen uptake during the last minute of exercise, expressed as millilitre per kilogram of body weight per minute. Oxygen consumption at the ventilatory (anaerobic) threshold was identified as the oxygen uptake before the systematic increase in the ventilatory equivalent for oxygen, without a concomitant increase in the ventilatory equivalent for carbon dioxide, together with the V-slope method. Echocardiographic measurement of left ventricular ejection fraction was performed at rest in all studied patients on the same day that the cardiopulmonary exercise test was conducted.
Calculations and statistical analysis
The cardiac power output was calculated from the product of cardiac output and mean arterial pressure using the following equation (18): CPO = (QT × MAP) × K, where CPO is cardiac power output in Watts (W), QT is cardiac output in litre per minute, MAP is mean arterial pressure in mmHg, and K is the conversion factor (2.22×10−3). The ventilatory response to exercise was calculated by linear regression analysis using the values of minute ventilation and carbon dioxide output as previously described.12 Circulatory power was calculated as the product of oxygen consumption and systolic blood pressure, expressed in millimetres of mercury litre per minute.15
All statistical analysis was carried out using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Prior to statistical analysis, data were checked for univariate and multivariate outliers using standard Z-distribution cut-offs and Mahalanobis distance tests, respectively. Normality of distribution was assessed using a Kolmogorov–Smirnov test. To test differences in measured variables between the patients with moderate-to-severe heart failure, those implanted and explanted with LVAD, a one-way analysis of variance was used. To identify the groups that differed significantly from one another, a post-hoc Tukey's test was performed. The relationship between peak cardiac power output and selected exercise-derived variables was assessed using Pearson's product moment coefficient of correlation. The meaningfulness of the coefficient of correlation was evaluated by calculating the coefficient of determination (R2). Statistical significance was indicated if P< 0.05. All data are presented as means ± SD unless otherwise indicated.
Results
There were non-significant differences in age and body mass index between patients with moderate-to-severe heart failure, implanted and explanted LVAD patients (P> 0.05), whereas LVEF was significantly higher in LVAD implanted and explanted compared with heart failure patients (P< 0.05) (Table 1). Although resting cardiac output and stroke volume were higher in LVAD-implanted than in heart failure patients (5.5 ± 2.1 vs. 4.1 ± 1.2 L/min, P< 0.05; and 76.6 ± 21.2 vs. 56.8 ± 20.8 mL/beat, P< 0.05), cardiac power output and other haemodynamic and metabolic measurements were not significantly different between the groups.
At peak exercise, however, cardiac power output in implanted and explanted LVAD patients was significantly higher compared with heart failure patients (P< 0.05). Oxygen consumption at anaerobic threshold and at peak exercise in explanted LVAD patients was significantly greater than that in implanted LVAD and heart failure patients (P< 0.05). Ventilatory efficiency slope was significantly lower in explanted and implanted LVAD patients compared with heart failure patients (P< 0.05). Peak circulatory power was however significantly lower in patients with heart failure and implanted compared with explanted LVAD patients (P< 0.01). Peak exercise haemodynamic, gas-exchange, and ventilatory variables are presented in Table 2.
| Variable | Heart failure | Implanted LVAD | Explanted LVAD |
|---|---|---|---|
| (n = 20) | (n = 18) | (n = 16) | |
| Cardiac power output (W) | 1.90 ± 0.45 | 2.37 ± 0.55* | 3.39 ± 0.61****,****** |
| Cardiac output (L/min) | 9.1 ± 2.1 | 12.4 ± 2.2* | 14.6 ± 2.9**** |
| Heart rate (b.p.m.) | 126.3 ± 27.3 | 142.2 ± 27* | 163 ± 18.1**** |
| Stroke volume (mL/beat) | 71.1 ± 14.1 | 87.2 ± 16.4** | 89.4 ± 15.1**** |
| Systolic blood pressure (mmHg) | 123.2 ± 22.1 | 110.7 ± 24.1 | 137.8 ± 22.4****** |
| Diastolic blood pressure (mmHg) | 75.0 ± 12.6 | 67.7 ± 11.5 | 76.6 ± 12.7 |
| Mean arterial blood pressure (mmHg) | 95.9 ± 16.2 | 87.4 ± 14.5 | 102.8 ± 15.0***** |
| Oxygen uptake (mL/min) | 1384 ± 375 | 1592 ± 554* | 2511 ± 468****,***** |
| Oxygen uptake (mL/kg/min) | 15.8 ± 5.8 | 19.8 ± 4.1* | 28.2 ± 5.0****,****** |
| Anaerobic threshold (mL/min) | 948 ± 381 | 1166 ± 246 | 1828 ± 418****,***** |
| Anaerobic threshold (mL/kg/min) | 11.2 ± 4.5 | 14.7 ± 3.1* | 21.4 ± 4.9****,****** |
| Circulatory power (mmHg L/min) | 173 ± 49 | 196 ± 56 | 334 ± 56****,****** |
| Carbon dioxide production (mL/min) | 1591 ± 378 | 1846 ± 401 | 2837 ± 550****,***** |
| Respiratory exchange ratio | 1.14 ± 0.12 | 1.15 ± 0.14 | 1.12 ± 0.11 |
| Minute ventilation (L/min) | 53.7 ± 10.8 | 62.2 ± 17.7* | 88.6 ± 28.0****,***** |
| Ventilatory efficiency slope | 35.8 ± 7.8 | 30.9 ± 9.1* | 29.3 ± 5.2*** |
- * P< 0.05
- ** P< 0.01, implanted LVAD vs. heart failure patients.
- *** P< 0.05
- **** P< 0.01, explanted LVAD vs. heart failure patients.
- ***** P< 0.05
- ****** P< 0.01, explanted LVAD vs. implanted LVAD patients.
When all data were combined (n= 54), peak cardiac power output was strongly correlated with peak oxygen consumption (r = 0.87, R2= 0.76, P< 0.01), anaerobic threshold (r = 0.79, R2= 62, P< 0.01), and circulatory power (r = 0.82, R2= 0.67, P< 0.01). Modest negative correlation was found between peak cardiac power output and ventilatory efficiency slope (r = −0.51, R2= −0.26, P< 0.01).
Figures 123–4 show subgroup analysis of the relationship between peak cardiac power and peak oxygen consumption, anaerobic threshold, peak circulatory power, and ventilatory efficiency slope in patients with heart failure, those implanted and explanted with an LVAD.
Discussion
The therapy with LVADs has been shown to improve prognosis, haemodynamics, end-organ function, quality of life, functional capacity, and diabetic control in patients with severe heart failure.1,8,22 The present study is the first to examine the relationship between peak cardiac power output, a direct indicator of overall cardiac function (i.e. cardiac pumping capability)16,17 and selected exercise-derived prognostic indicators in implanted and explanted (recovered) LVAD patients.
Major findings suggest that the relationship between peak cardiac power output and peak oxygen consumption, anaerobic threshold, circulatory power, and ventilatory efficiency is only mild-to-moderate in implanted LVAD patients and those with moderate-to-severe heart failure, suggesting that these variables are poor indicators of cardiac function and cardiac pumping capability per se. On the other hand, in explanted (recovered) LVAD patients the relationship between peak cardiac power and other evaluated exercise-derived variables is high, suggesting these to be excellent surrogates of peak cardiac power output.
Peak oxygen consumption is the most frequently analysed cardiopulmonary exercise test parameter and a ‘gold’ standard among other cardiopulmonary exercise measures in heart failure evaluation and prognosis. Specifically, it has been used to assess functional capacity, evaluate responses to medical treatment, assess progression of disease process, and to determine prognosis.5–7 The relationship between peak cardiac power output and peak oxygen consumption has been subject to numerous other investigations. Similarly as in explanted LVAD patients, peak cardiac power output was highly correlated with peak oxygen consumption in patients with mild-to-moderate heart failure.23,24 A strong relationship between peak cardiac power output and peak oxygen consumption should not be surprising, as the flow generating capacity of the heart, namely cardiac output, determines both variables. Measurement of cardiac output is not as straightforward as measurement of oxygen consumption, particularly during exercise, and therefore approximations of cardiac power output have been sought. Cohen-Solal et al.15 proposed circulatory power as a new ‘surrogate’ of cardiac power output that has recently been shown to be an excellent predictor of outcome in chronic heart failure patients.14 Not surprisingly, peak circulatory power correlated well with peak cardiac power in explanted LVAD patients. A similar finding was previously reported in patients with mild-to-moderate chronic heart failure.25 Peak exercise cardiac power was greater in LVAD explanted than in LVAD implanted and heart failure patients. A number of factors may account for this difference and it is likely that ventricular pressure and volume unloading provided while patients were supported with an LVAD led to reverse remodelling on genetic, biochemical, histological, and functional levels.1,3,4 Additionally, higher intensity and duration of exercise as well as a delay in onset of anaerobic metabolism could potentially stimulate higher cardiac response in LVAD-explanted compared with LVAD-implanted and heart failure patients. Taken together, these factors may explain differences in the strength of the relationship between peak exercise cardiac function and other exercise-derived variables between the LVAD-explanted, LVAD-implanted, and heart failure patients.
Converse to explanted, in implanted LVAD patients and those with moderate-to-severe heart failure, peak oxygen consumption and its derivate circulatory power, were not good predictors of cardiac pumping capability. That peak oxygen consumption does not reflect the severity of cardiac dysfunction in heart transplant candidates and chronic heart failure patients have been shown by previous studies.26,27 This finding may be explained by the fact that peak oxygen consumption, in addition to central (cardiac) factors, may also be limited by peripheral (e.g. muscle conditioning, blood flow abnormalities, vasodilatory capacity, skeletal muscle biochemistry) and ventilatory factors (pulmonary pressure, physiological dead space, ventilation-perfusion mismatch, respiratory control, breathing pattern).28 Specifically, in implanted LVAD patients, the effect of the device on exercise cardiac output is unknown,20 and perhaps these patients with deconditioned skeletal muscle were not able to extract supplied oxygen.
The relationship between minute ventilation and carbon dioxide production, called ventilatory efficiency, is one of the cardiopulmonary exercise test parameters that appear to have important clinical value in heart failure patients.7,12,13 Ventilatory efficiency slope was inversely related to cardiac power output in explanted LVAD patients. A similar finding that ventilatory efficiency slope is inversely related to cardiac function has been previously reported.29 However, the relationship between the two variables does not warrant the conclusion that ventilatory efficiency slope predicts cardiac pumping capability.
Finally, anaerobic threshold, a measure that is independent of patient motivation, has been used as an index of exercise tolerance and functional capacity in chronic heart failure.30,31 Additionally, its importance when prioritizing patients for heart transplantation has also been demonstrated.11 Previous studies have shown that anaerobic threshold is highly correlated with peak oxygen consumption.5,27,32 Although anaerobic threshold was well related to cardiac function when all data were combined (N = 54), subgroup analysis demonstrated a mild-to-moderate relationship between peak cardiac power and anaerobic threshold. This discrepancy may be explained by different mean and wide range values of anaerobic threshold between the three groups of patients, resulting in a steeper slope when all data were combined.
In the present study certain limitations should be considered. The study sample size was relatively small, particularly within each subgroup of patients. The study was limited to men and it remains an issue whether the investigated relationships would be similar in women.
In conclusion, the present study suggests that powerful exercise-derived prognostic indicators, including peak oxygen consumption, anaerobic threshold, circulatory power, and ventilatory efficiency slope demonstrate limited capacity to reflect cardiac organ function in patients treated with LVADs. This is becoming particularly important as the survival of patients following LVAD implantation improves and hence patient's outcome is likely to be judged more by changes in functional capacity and quality of life, particularly when deciding the timing of implantation. Hence, for upcoming trials to evaluate whether LVADs should be implanted at an earlier stage in heart failure, a better understanding of which parameters accurately reflect cardiac functional capacity is very important. The imperfect correlation between cardiac power and other exercise-derived variables may suggest that exercise performance of LVAD-implanted patients may be limited by factors other than cardiac. In the absence of direct measurements of cardiac organ function/dysfunction and its functional reserve, clinicians need to interpret exercise-derived prognostic indicators with caution.
Acknowledgements
The authors thank to Mr Robert Bougard, Mrs Gay Donovan, and Dr David Nunan for their contribution in the collection of data.
Funding
This study was funded by the Research Centre for Health Studies at Buckinghamshire New University. D.J. received a bursary to undertake this study as part of his PhD thesis. Royal Brompton and Harefield NHS Foundation Trust received an education grant to study recovery in patients receiving the Harefield Recovery protocol from Thoratec Corporation.
Conflict of interest: none declared.




