Myocardial gene expression alterations in peripheral blood mononuclear cells of patients with idiopathic dilated cardiomyopathy
Abstract
Aims
To assess cardiac gene expression in peripheral blood cells of patients with idiopathic dilated cardiomyopathy (IDCM) and its relationship to echocardiographic left ventricular (LV) function.
Methods and results
A complete echocardiographic study and blood sampling were performed in 65 consecutive stable IDCM patients with LV ejection fraction (LVEF) 31.76 ± 10.07% and chronic mild to moderate heart failure (NYHA functional class II to III) for ≥9 months. Blood samples from 19 healthy individuals were included for comparison. Transcript levels of myocardin, GATA4, alpha- and beta-myosin heavy chain (MHC), sarcoplasmic reticulum calcium ATPase 2 (SERCA2), and phospholamban were determined by quantitative real-time reverse transcription-polymerase chain reaction. Myocardin (24.88 ± 4.93 vs. 3.98 ± 1.12, P = 0.0048) and GATA4 (17.85 ± 4.85 vs. 0.45 ± 0.15, P = 0.0069 × 10−5) were upregulated in IDCM patients compared with controls, whereas SERCA2 (5.11 ± 0.42 vs. 8.93 ± 1.07, P = 0.001) was downregulated. In IDCM patients, myocardin (r = 0.279, P = 0.025), GATA4 (r = 0.314, P = 0.011), beta-MHC (r = 0.444, P=0.0002), and alpha-MHC (r = 0.272, P = 0.034) showed positive correlations, whereas SERCA2 (r = −0.264, P = 0.034) exhibited a negative correlation with LVEF. Patients with elevated LV filling pressures had lower myocardin (15.06 ± 3.10 vs. 43.12 ± 12.03, P = 0.048), GATA4 (8.96 ± 2.17 vs. 34.38 ± 12.60, P = 0.026), beta-MHC (10.59 ± 4.05 vs. 16.43 ± 4.91, P = 0.013), and alpha-MHC (0.27 ± 0.08 vs. 0.79 ± 0.20, P = 0.033) and higher SERCA2 (5.65 ± 0.54 vs. 3.90 ± 0.61, P = 0.037) levels. Patients with atrial fibrillation (AF) had higher SERCA2 levels compared with sinus rhythm patients (6.75 ± 0.84 vs. 4.54 ± 0.45, P = 0.017).
Conclusion
Our data indicate that cardiac gene expression alterations in peripheral blood cells of IDCM patients may reflect alterations in LV function, whereas the presence of AF may be associated with increased SERCA2 levels in these patients.
Introduction
Heart failure (HF) is characterized by adverse left ventricular (LV) remodelling and reduced contractile function associated with an altered gene expression profile. Dilated cardiomyopathy (DCM) is a common form of heart muscle disease with an estimated prevalence of 1:2500; it is the third most common cause of HF and the most frequent reason for heart transplantation. It is characterized by ventricular enlargement and impaired contraction with normal wall thickness and is usually identified at the time it produces severe limiting symptoms and disability due to HF. The DCM phenotype has a broad range of primary and secondary causes and is characterized by high mortality.1 It has, therefore, become increasingly important to identify high-risk patients at earlier stages. Many studies have attempted to assess which data obtained at the time of diagnosis provide the best prognostic indices.
The role of echocardiography is well established in DCM patients, for both screening and prognostication, and accumulating data have shown that pulsed wave Doppler mitral inflow velocity parameters and filling patterns correlate better with LV filling pressures and prognosis than LV ejection fraction (LVEF).2 A restrictive LV filling pattern is common in DCM, is associated with more severe disease, and is a powerful indicator of increased mortality risk and need for heart transplantation. Furthermore, recent studies have examined the prognostic role of the E/E′ ratio, which emerges as a highly reproducible and reliable surrogate of LV filling pressures.2
At the molecular level, adverse cardiac remodelling is characterized by the re-employment of developmental transcription factors and activation of the ‘foetal’ cardiac gene program, including an isoform switch in the myosin heavy chain (MHC).3,4 Other molecular changes include alterations in the expression of genes encoding excitation–contraction (EC) coupling, such as sarcoplasmic reticulum calcium ATPase 2 (SERCA2) and phospholamban (PLB), resulting in impaired calcium cycling.5 These changes seem to represent the basic molecular mechanisms underlying LV dysfunction and HF. In this regard, changes in myocardial gene expression have been considered as confirmatory evidence of pathological remodelling and reversal of remodelling in HF.4
During the last decade, microarray technology using endomyocardial biopsies has developed new sophisticated transcriptomic biomarkers, defining a molecular profile useful in the prediction of disease aetiology, outcome, and responsiveness to therapy: so called ‘personalized’ medicine.6 However, this represents a highly invasive procedure. The need for improved, non-invasive diagnostic tools becomes more urgent with the growth of the ageing population with increasing cardiovascular disease risk factors. As cardiovascular tissue specimen collection is limited by practical considerations, the need for reliable non-invasive biomarkers from a substitute tissue that can report on disease states has grown tremendously. Peripheral blood is an easily obtained tissue compared with a cardiac biopsy sample and has been suggested as a powerful substitute material for monitoring cardiovascular disease states, as it contains a number of circulating cell types, including stem cells, that are mechanistically associated with myocardial and vascular disease processes and thus can probably act as an indicator of disease severity. Changes within the body may trigger disease-specific changes within the transcriptome of blood cells that can then be used as biomarkers for diagnostic purposes.7 It has been shown that 84.2% of the genes known to be expressed in the heart muscle are also expressed in blood.8 A recent genome-wide analysis study in the peripheral blood of patients with chronic HF (CHF) identified a gene expression profiling that distinguished between CHF patients and controls.9 The use of peripheral blood as a sensor substitute tissue in cardiovascular disease would allow us to evaluate gene expression changes at any disease stage, whereas all the knowledge we have obtained from biopsy-based studies comes from advanced stages of heart disease in a limited sample.
In the present study, we evaluated gene expression levels of selected genes related to HF and contractile function in the peripheral blood cells of idiopathic dilated cardiomyopathy (IDCM) patients in relation to echocardiographic indices of LV function. We selected developmental transcription factors (early myocardial markers), myocardin and GATA4,3 as well as genes participating in the contractile apparatus, alpha- and beta-MHC,10 and EC coupling, SERCA2 and PLB.5
Methods
Study population
Sixty-five consecutive IDCM patients, mean age 60.9 ± 9.7 years, 60 men and 5 women, were enrolled from the HF clinic of our hospital. Blood samples from 19 healthy controls with the same age and gender distribution were obtained from the blood bank of our hospital. All patients had an echocardiographic diagnosis of DCM, defined as LV end-diastolic dimension >55 mm with LVEF <45% and chronic mild to moderate HF (NYHA functional class II to III) for ≥ 9 months. All patients underwent left cardiac catheterization and coronary angiography before their inclusion in the study. Exclusion criteria were: the presence of significant coronary artery disease, lumen stenosis >50% of any coronary artery or prior myocardial infarction (MI), severe primary valve disease, hypertrophic or restrictive cardiomyopathy, long-standing or uncontrolled systemic hypertension, chronic systemic disease involving the heart muscle, myocarditis, thyroid disease, drug abuse, administration of adriamycin, and HIV disease. All patients were on optimal medication including diuretics, angiotensin-converting enzyme inhibitors, digoxin, and beta-blockers and had been clinically stable for at least 1 month. Plasma levels of brain natriuretic peptide (BNP) (detection limit 5 fmol/mL) were measured by ELISA according to the manufacturer's instructions (Biomedica, Wien, Austria).
The present study conforms to the Declaration of Helsinki for the use of human tissue or subjects. The study was approved by the Hospital Ethics Committee and all patients gave written informed consent to their participation in the study. Baseline data are presented in Table Table 1.
| Patients (n = 65) | Controls (n = 19) | |
|---|---|---|
| Age (years) | 60.9 ± 9.7 | 57.89 ± 7.32 |
| Males/females | 60/5 | 17/2 |
| Sinus rhythm/atrial fibrillation | 47/18 | 19/0 |
| Restrictive/non-restrictive filling pattern | 19/46 | |
| Interventricular septum in diastole (mm) | 10.05 ± 1.07 | |
| Ventricular posterior wall in diastole (mm) | 9.96 ± 1.22 | |
| Left ventricular end-diastolic diameter (mm) | 62.25 ± 6.95 | |
| Left ventricular end-systolic diameter (mm) | 48.95 ± 9.93 | |
| Left ventricular end-diastolic volume (mL) | 156.14 ± 49.62 | |
| Left ventricular end-systolic volume (mL) | 108.62 ± 47.91 | |
| Left ventricular ejection fraction (%) | 31.76 ± 10.07 | |
| Left atrial diameter (mm) | 44.52 ± 6.21 | |
| Aortic diameter (mm) | 32.65±3.72 | |
| E-wave deceleration time (ms) | 193.79 ± 69.81 | |
| Peak early transmitral filling velocity (E) (cm/s) | 74.36 ± 26.05 | |
| Peak late transmitral filling velocity (A) (cm/s) | 65.58 ± 24.38 | |
| E/A | 1.28 ± 0.86 | |
| Mean peak systolic velocity (S′) (cm/s) | 4.41 ± 1.14 | |
| Early diastolic tissue velocity (E′) (cm/s) | 4.74 ± 1.96 | |
| Late diastolic tissue velocity (A′) (cm/s) | 5.67 ± 1.83 | |
| E/E′ | 17.38 ± 8.95 |
- a Data are presented as mean ± SD.
Echocardiography
Standard M-mode, two-dimensional echocardiography, and Doppler measurements of LV function were performed using a Vivid 7 (General Electric, Horten, Norway) ultrasound device with a 1.5–3.6 MHz wide angle phased-array transducer (M4S) according to the recommendations of the American Society of Echocardiography and all examinations were digitally stored for off-line analysis as the mean of three cardiac cycles.2 Pulsed wave Doppler recordings from the mitral inflow were obtained in the apical four-chamber view to assess LV filling dynamics and the following parameters were measured: (i) peak early (E-wave) and late (A-wave) transmitral filling velocities in centimetres per second; (ii) E/A ratio; and (iii) deceleration time of the E (DTE) velocity in milliseconds (from peak E velocity to baseline). Pulsed wave tissue Doppler imaging was performed in the apical four-chamber view to acquire mitral annular velocities. The sample volume was set at 5 × 5 mm and placed at or 1 cm within the septal and lateral insertion sites of the mitral leaflets, and systolic (S′), early diastolic (E′), and late diastolic (A′) velocities were acquired. Data from three consecutive cardiac cycles were transferred to a workstation for further analysis using EchoPAC software (GE, Norway). The final value represented the average of both sites. From the mitral flow and annular velocities, the ratios of annular E′/A′ and the mitral inflow velocity E to tissue Doppler E′ (E/E′) were computed.2 For the estimation of LV filling pressures, the mitral inflow pattern was used. A restrictive filling pattern with E/A ≥ 2 and DTE < 150 ms predicts high LV filling pressures, whereas patients with E/A < 1 and E ≤ 50 cm/s were assumed to have normal pressures. In all other cases that were in the gray zone with either E/A ≥ 1 and <2 or E/A < 1 but E > 50 cm/s additional use of the E/E′ ratio was applied according to the recently published algorithms.2 An E/E′ < 8 predicts normal, whereas an E/E′ > 15 predicts high filling pressures. On the basis of the above criteria, the study population was divided into two groups: those with normal (Group A) and those with high (Group B) filling pressures. In patients with atrial fibrillation (AF) and DCM, mitral DTE (≤150 ms) and the E/E′ ratio ≥11 were used as a surrogate of increased LV filling pressures.
RNA isolation and quantitative reverse transcription-polymerase chain reaction
Blood samples were collected into EDTA (ethylenediaminetetraacetic acid) collection tubes. Peripheral blood mononuclear cells (PBMCs) were isolated by Histopaque-ficoll (SIGMA) centrifugation, total RNA was isolated using the TRI-Reagent (Ambion), and 1 µg RNA was reverse-transcribed with oligo-(dT) using the Reverse Transcription System (Promega) in 20 µL reactions. Measurements of mRNA levels were performed by real-time reverse transcription-polymerase chain reaction using the STRATAGENE Mx3000P Detection System. Polymerase chain reaction assays were performed in 1 µL of cDNA template using the SYBR Green PCR Master Mix (Bio-Rad). All samples were performed in triplicate. The standard curve method was used for absolute quantification of the amplification products and specificity was determined by performing a melting curve analysis.11 Standard curves for expression of each gene were generated by serial dilution of known quantities of cDNA template. The housekeeping gene GAPDH (glyceraldehyde-3-phosphate-dehydrogenase) was used as an endogenous reference gene. Primers for alpha-MHC were 5′-GAAGGAGTTTGACATTAATCAGC-3′ and 5′-CAGC TTCTCCACCTTAGCC-3′, for SERCA2 5′-T GTGTAACGCCCTCAACAGC-3′ and 5′-GAG AATCACGGGCAAGGAGAT-3′, and for PLB 5′-TGATGATCACAGCTGCCAAG-3′ and 5′-CTGAGCGAGTGAGGTATTGGA-3′. Primer sequences for myocardin, GATA4, beta-MHC, and GAPDH as well as the experimental design strategy to achieve specificity were as previously described.12
Statistics
Correlations between continuous variables were assessed using the Pearson correlation coefficient. The significance of gene expression differences between groups was evaluated by a two-tailed Mann–Whitney test. All tests were two-sided, and analysis was performed with a commercially available statistical package (SPSS for Windows 13.0, Chicago, IL, USA). In order to avoid false-positive results, P-values less than 0.01 were considered to be statistically significant, whereas P-values less than 0.05 were considered to be indicative.
Results
The expression levels of the early myocardial marker genes myocardin and GATA4, as well as the contractile related genes alpha-MHC, beta-MHC, SERCA2, and PLB, were studied in PBMCs of patients with IDCM (n = 65) in relation to echocardiographic parameters of systolic and diastolic function. Gene expression levels were also examined in PBMCs of 19 healthy controls for comparison.
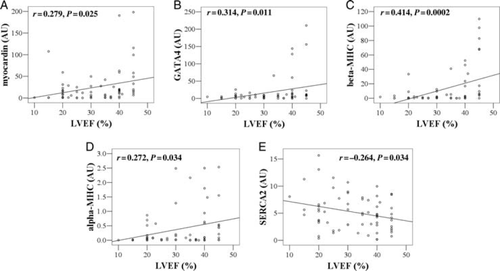
Myocardin (24.88 ± 4.93 vs. 3.98 ± 1.12, P = 0.0048) and GATA4 (17.85 ± 4.85 vs. 0.45 ± 0.15, P = 0.0069 × 10−5) were upregulated in IDCM patients compared with controls, whereas SERCA2 (5.11 ± 0.42 vs. 8.93 ± 1.07, P = 0.001) was downregulated (Table Table 2). In IDCM patients, we found indicative or significant positive correlations between myocardin (r = 0.279, P = 0.025), GATA4 (r = 0.314, P = 0.011), beta-MHC (r = 0.444, P=0.0002), and alpha-MHC (r = 0.272, P = 0.034) gene expression and LVEF (Figure Figure 1A–D, respectively). An indicative negative correlation was observed between SERCA2 expression and LVEF (r = −0.264, P = 0.034) (Figure Figure 1E). Correlations with other echocardiographic variables are shown in Table Table 3. Alpha-MHC expression was positively correlated with mean peak systolic tissue velocity S′ (r = 0.343, P = 0.005) and GATA4 showed an indicative correlation with S′ (r = 0.273, P = 0.024). Significant positive correlations between myocardin (r= 0.374, P = 0.002) and GATA4 (r = 0.368, P = 0.003), and DTE were also observed (Table Table 3). PLB expression levels did not show any significant correlation with echocardiographic parameters of systolic and diastolic LV function.
| Gene | Controls (n = 19) | Patients (n = 65) | P-value* |
|---|---|---|---|
| Myocardin | 3.98 ± 1.12 | 24.88 ± 4.93 | 0.0048 |
| GATA4 | 0.45 ± 0.15 | 17.85 ± 4.85 | 0.0069 × 10−5 |
| Alpha-MHC | 0.15 ± 0.05 | 0.44 ± 0.09 | 0.782 |
| Beta-MHC | 9.55 ± 3.07 | 12.34 ± 3.05 | 0.055 |
| SERCA2 | 8.93 ± 1.07 | 5.11 ± 0.42 | 0.001 |
- a MHC, myosin heavy chain; SERCA, sarcoplasmic reticulum calcium ATPase.
- * Two-tailed descriptive P-values (asymptomatic significance) from Mann–Whitney test for independent groups.
| Myocardin | GATA4 | Beta-MHC | Alpha-MHC | SERCA2 | PLB | BNP† | ||
|---|---|---|---|---|---|---|---|---|
| LA (mm) | r | −0.278* | −0.217 | −0.042 | −0.270* | 0.007 | −0.354** | 0.276 |
| P | 0.025 | 0.083 | 0.744 | 0.040 | 0.957 | 0.008 | 0.094 | |
| LVPW (mm) | r | 0.087 | 0.112 | 0.300* | 0.015 | 0.072 | 0.101 | 0.074 |
| P | 0.448 | 0.328 | 0.010 | 0.907 | 0.540 | 0.437 | 0.635 | |
| LVESD (mm) | r | −0.165 | −0.221 | −0.298* | −0.280* | 0.148 | −0.182 | 0.421** |
| P | 0.212 | 0.093 | 0.023 | 0.035 | 0.264 | 0.180 | 0.009 | |
| LVESV (mL) | r | −0.104 | −0.163 | −0.262* | −0.202 | 0.213 | −0.143 | 0.378* |
| P | 0.442 | 0.224 | 0.049 | 0.140 | 0.112 | 0.307 | 0.021 | |
| LVEF (%) | r | 0.279* | 0.314* | 0.444** | 0.272* | −0.264* | 0.084 | −0.390* |
| P | 0.025 | 0.011 | 0,0002 | 0.034 | 0.034 | 0.533 | 0.012 | |
| DTE (ms) | r | 0.374** | 0.368** | 0.089 | 0.189 | −0.080 | 0.231 | −0.331* |
| P | 0.002 | 0.003 | 0.469 | 0.129 | 0.513 | 0.076 | 0.032 | |
| S′ (cm/s) | r | 0.158 | 0.273* | 0.124 | 0.343** | −0.200 | 0.112 | −0.388* |
| P | 0.198 | 0.024 | 0.316 | 0.005 | 0.103 | 0.402 | 0.011 | |
| E (cm/s) | r | −0.156 | −0.209 | −0.052 | −0.237 | 0.208 | −0.153 | 0.344* |
| P | 0.200 | 0.085 | 0.673 | 0.056 | 0.086 | 0.242 | 0.026 | |
| A (cm/s) | r | 0.245 | 0.395** | −0.048 | −0.011 | −0.001 | 0.193 | −0.090 |
| P | 0.074 | 0.003 | 0.731 | 0.937 | 0.995 | 0.194 | 0.630 | |
| E′ (cm/s) | r | 0.082 | 0.087 | 0.205 | 0.063 | −0.034 | 0.179 | −0.271 |
| P | 0.504 | 0.478 | 0.093 | 0.619 | 0.780 | 0.174 | 0.082 | |
| A′ (cm/s) | r | 0.205 | 0.128 | 0.096 | 0.353** | −0.335* | −0.066 | −0.460** |
| P | 0.123 | 0.337 | 0.476 | 0.008 | 0.010 | 0.651 | 0.004 | |
| E/A | r | −0.131 | −0.256 | 0.017 | −0.046 | 0.052 | −0.159 | 0.333 |
| P | 0.344 | 0.061 | 0.904 | 0.750 | 0.709 | 0.286 | 0.067 | |
| E′/A′ | r | −0.096 | −0.059 | 0.247 | 0.150 | 0.251 | 0.175 | 0.049 |
| P | 0.485 | 0.666 | 0.072 | 0.288 | 0.065 | 0.234 | 0.780 | |
| E/E′ | r | −0.181 | −0.186 | −0.175 | −0.192 | 0.209 | −0.199 | 0.529** |
| P | 0.149 | 0.137 | 0.166 | 0.135 | 0.098 | 0.141 | 0.0004 |
- a MHC, myosin heavy chain; SERCA, sarcoplasmic reticulum calcium ATPase; BNP, brain natriuretic peptide; LA, left atrial diameter; LVPW, left ventricular posterior wall in diastole; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; DTE, E-wave deceleration time; S′, mean peak systolic velocity; E, peak early transmitral filling velocity; A, peak late transmitral filling velocity; E′, early diastolic tissue velocity; A′, late diastolic tissue velocity.
- b Pearson correlations (r), Sig. (two-tailed) (P) are presented.
- * Correlation is significant at the 0.05 level (two-tailed).
- ** Correlation is significant at the 0.01 level (two-tailed).
- † Correlations of echocardiographic variables with plasma BNP levels (pg/mL) are presented for comparison.
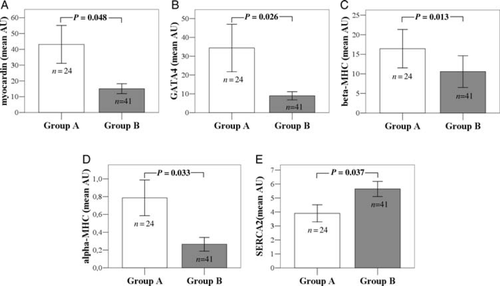
Patients with elevated LV filling pressures (Group B) compared with patients with normal filling pressures (Group A) appeared to have lower myocardin (15.06 ± 3.10 vs. 43.12 ± 12.03, P = 0.048), GATA4 (8.96 ± 2.17 vs. 34.38 ± 12.60, P = 0.026), beta-MHC (10.59 ± 4.05 vs. 16.43 ± 4.91, P = 0.013), and alpha-MHC (0.27 ± 0.08 vs. 0.79 ± 0.20, P = 0.033) and higher SERCA2 (5.65 ± 0.54 vs. 3.90 ± 0.61, P = 0.037) transcript levels in peripheral blood cells (Figure Figure 2A–E, respectively). Patients with elevated LV filling pressures had increased BNP levels compared with patients with normal LV filling pressures (141.06 ± 29.24 vs. 71.36 ± 28.74, P = 0.008).
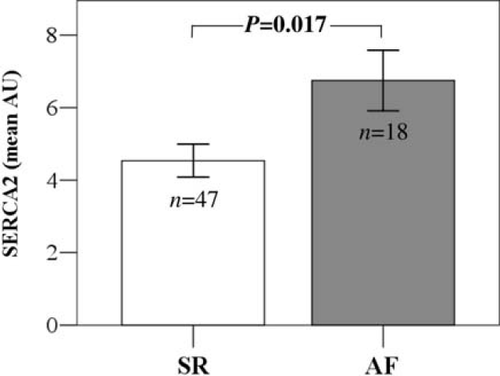
An interesting observation was that SERCA2 expression levels tended to be higher in patients with AF when compared with patients in sinus rhythm (SR) (6.75 ± 0.84 vs. 4.54 ± 0.45, P = 0.017) (Figure Figure 3). The trend towards higher SERCA levels in AF compared with SR patients remained even when we compared only patients with LVEF ≤ 35% (7.41 ± 1.05 vs. 5.14 ± 0.67, P = 0.049) or only patients with elevated filling pressures (Group B) (6.76 ± 0.89 vs. 4.83 ± 0.65, P = 0.092).
Discussion
We have demonstrated that myocardial gene expression alterations in the peripheral blood cells of IDCM patients seem to follow functional echocardiographic parameters. Our data provide some evidence to support the hypothesis that gene expression in peripheral blood cells may reflect the disease severity, suggesting that peripheral blood could possibly be a good substitute tissue for gene expression analysis, even in non-end-stage cardiovascular disease.
Early cardiac genes and left ventricular function
Previous studies have shown that myocardin expression is augmented in failed hearts compare with non-failed.13 We also found increased myocardin expression in peripheral blood cells in patients compared with healthy individuals. However, this is a black and white comparison of failed to non-failed status. Probably, myocardin expression is increased in failed hearts compared with normal, but our data indicate that increased expression of myocardin, at least in the peripheral blood of IDCM patients, appears to accompany a less severe stage of the disease. We observed a positive correlation between myocardin expression and LVEF as well as DTE, indicating that increased expression of myocardin may reflect better LV function. Our observation is in agreement with a previous study that showed that forced expression of myocardin in mesenchymal stem cells before transplantation was associated with better LV function.14 The same observations were also true for GATA4, which also shows increased expression in patients compared with healthy individuals and in patients correlated positively with LVEF and DTE. In addition, patients showing LV diastolic dysfunction, as estimated by elevated LV filling pressures, appear to have lower myocardin and GATA4 transcript levels when compared with patients with normal LV filling pressures, indicating that reduced expression of myocardin and GATA4 may reflect diastolic dysfunction. Our GATA4 results are in agreement with recent animal observations. Transgenic cardiac-specific reduced GATA4 expression was associated with cardiac dilatation and HF in mice.15 Myocardin and GATA4 transcription factors are part of the so called foetal gene program.3 Partial or complete return to the foetal gene profile is a common feature of the stressed heart.4 Return to the foetal program is most probably an adaptive process associated with survival, rather than destruction of the heart.4 Our data showing that increased myocardin and GATA4 gene expression may reflect better heart function support this strong hypothesis.
Early cardiac genes in peripheral blood are possibly expressed in circulating stem–progenitor cells that reside in the bone marrow, and alterations in the expression levels of such genes most possibly reflect alterations in these cell populations. Previous studies reported mobilization of bone marrow-derived stem–progenitor cells in peripheral blood after MI, associated with LVEF improvement.16 In agreement with this, we found increased expression of the early cardiac marker genes myocardin and GATA4 in patients with better LV function, as defined by LVEF as well as LV filling pressures. Possibly, alterations of bone marrow stem–progenitor cell populations, indicated by early cardiac gene expression, may contribute to better myocardial function. There is no direct proof that endogenous circulating bone marrow stem–progenitor cells contribute to myocardial function, but clinical trials have shown that bone marrow cells injected into the myocardium improve LVEF in patients with severe HF,17 whereas it has also been reported that circulating CD34+ progenitor cell populations are increased in DCM patients18 and intracoronary administration of bone-marrow progenitor cells improved LV contractility in non-ischaemic DCM patients.19 The existence of an endogenous native, although inadequate, cardiac repair response mechanism through cardiac progenitor cells (CPCs) in human failing hearts, has been hypothesized.20 Such a mechanism, if it exists, could provide the impetus for the development of new alternative therapeutic strategies for HF. Nevertheless, the fact that early cardiac marker gene expression alterations in peripheral blood cells seem to follow indices of myocardial function indicates that peripheral blood may possibly reflect the disease severity, and thus might be a good substitute tissue for gene expression analysis in IDCM.
Myosin heavy chain isoforms and left ventricular function
Myosin heavy chains, alpha and beta, are known to be downregulated in the human failing heart and an increase of the beta/alpha ratio is observed.10 In accordance with this, in our study, we observed that differences in alpha- and beta-MHC isoforms in the peripheral blood of patients with IDCM were correlated with echocardiographic parameters of contractile function. Decreased expression of alpha- and beta-MHC was mainly associated with LV dysfunction, as indicated by its positive correlation with LVEF. We also observed a positive correlation between alpha-MHC gene expression and peak systolic tissue velocity (S′), indicating that reduced expression of alpha-MHC is associated with systolic dysfunction. In addition, patients showing LV diastolic dysfunction, as estimated by elevated LV filling pressures, appear to have lower alpha- and beta-MHC transcript levels compared with patients with normal LV filling pressures, indicating that the reduced expression of alpha- and beta-MHC is rather associated with diastolic dysfunction.
The association of alpha- and beta-MHC expression levels in peripheral blood cells of IDCM patients with indices of LV function indicates that peripheral blood could possibly reflect the severity of the disease.
Sarcoplasmic reticulum calcium ATPase and left ventricular function
Sarcoplasmic reticulum calcium ATPase is known to be downregulated in end-stage HF in myocardial tissue and several previous studies indicate that deficiencies of SERCA function and regulation may be a significant factor in the pathogenesis of HF.5 In accordance with that, we also found decreased SERCA expression levels in peripheral blood cells in patients compared with healthy individuals. We also observed that patients with elevated LVEF appear to have lower SERCA levels in peripheral blood cells. In addition, we show that patients with LV diastolic dysfunction, as estimated by elevated LV filling pressures, appear to have higher SERCA transcript levels compared with patients with normal filling pressures, indicating that increased SERCA levels may reflect LV diastolic dysfunction. Our data are in accordance with the occurrence of a PLB null mutation and a missense mutation resulting in non-inhibition of SERCA in DCM patients.21
Sarcoplasmic reticulum calcium ATPase plays an important role in the relaxation of heart muscle as a component of the Ca2+ handling machinery. Changes of calcium handling affect cardiac EC coupling and could result in impairment of contractile function.5
We observe that increased SERCA levels in the peripheral blood of IDCM patients are possibly associated with contractile dysfunction. A major limitation of our study is that we do not know which cells contribute to the observed SERCA levels. It was recently shown that SERCA is also expressed in CPCs, playing an important role in their growth and regenerating potential.22 A possible explanation of our result is that increased SERCA levels in peripheral blood in more severe stages of the disease could probably be part of an adaptive survival mechanism.
Animal-based evidence shows that increased SERCA activity has a beneficial effect on contractile function;5 preclinical studies in large animals showed that transfer of SERCA can reverse cardiac dysfunction,23 and a multicenter clinical trial in humans is under way, trying to increase SERCA levels in HF patients (ClinicalTrials.gov identifier: NCT00454818).
Sarcoplasmic reticulum calcium ATPase and atrial fibrillation
In our study, we also report that SERCA expression levels were higher in patients with AF compared with patients in SR. The results of biochemical studies of changes in Ca2+ handling proteins in AF are inconsistent. Previous studies using atrial appendage tissue showed that SERCA transcript levels in AF compared with SR are either reduced or unaltered.24 However, these observations were not in concurrence with IDCM and HF. Yeh et al.,25 in an experimental model of HF in dogs, demonstrated significant changes in atrial cardiomyocyte calcium-handling proteins—including SERCA—resulting in sarcoplasmic reticulum calcium overload with subsequent abnormal automaticity and triggered activity, which could initiate or maintain AF. In addition, it was recently suggested that a greater sarcoplasmic reticulum Ca2+ load and a higher SERCA content in atrial myocytes may play an important role in the Ca2+-signalling dysfunction that contributes to the pathogenesis of AF.26
Abnormal calcium metabolism contributes to the development of AF in the setting of HF and vice versa.27 The severity of calcium cycling abnormalities correlates with the degree of ventricular dysfunction.24 Although AF in combination with HF has emerged as a new cardiovascular epidemic, associated with increased morbidity and mortality, the pathophysiological relationship between AF and HF has only been partially elucidated.28 Our study provide data for the first time that suggest a correlation between SERCA transcript levels in peripheral blood and the presence of AF in IDCM patients.
The observed increased SERCA levels in peripheral blood could possibly be part of an adaptive response mechanism of AF patients, trying to reverse contractile dysfunction. It has been shown that inhibition of SERCA prevents acceleration of SR by beta-adrenergic stimulation29 and that during AF-related Ca2+ overload conditions in vivo, reduced inhibition of SERCA helps to maintain a normal sarcoplasmic reticulum Ca2+ load in patients with chronic AF, despite the increased frequencies of Ca2+ sparks through leaky ryanodine channels.30 The complete molecular mechanism of electrical and contractile remodelling has not been completely elucidated and remains unclear, although a great deal has been learned.24,31
Further investigation of calcium-handling changes in AF and HF concurrence would give a more accurate explanation of the observed increased SERCA transcript levels in the peripheral blood of IDCM patients with AF and a better understanding of the natural history of concurrent AF and HF, helping to determine possible therapeutic interventions for these patients.
Conclusions
Cardiac gene transcript levels alterations in the peripheral blood cells of IDCM patients seem to follow indices of LV function as assessed using Doppler echocardiography. Increased levels of myocardin, GATA4, alpha-MHC, and beta-MHC and decreased levels of SERCA may reflect better LV function. Our results suggest that the presence of AF in IDCM patients may be associated with increased SERCA levels.
The findings of this study suggest that gene expression levels in peripheral blood cells of IDCM patients may reflect the disease severity. Therefore, peripheral blood could possibly be a good substitute tissue for gene expression analysis in cardiomyopathies. Further studies in larger populations will be needed to reinforce or refine our indicative observations.
Acknowledgements
We thank Gregory Chlouverakis, Associate Professor of Biostatistics, School of Medicine, University of Crete, for statistical assistance, and Philip Lees, Technical Editor of the Hellenic Journal of Cardiology, for help in editing the manuscript.
Conflict of interest: none declared.




