Assessing and grading congestion in acute heart failure: a scientific statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine
Abstract
Patients with acute heart failure (AHF) require urgent in-hospital treatment for relief of symptoms. The main reason for hospitalization is congestion, rather than low cardiac output. Although congestion is associated with a poor prognosis, many patients are discharged with persistent signs and symptoms of congestion and/or a high left ventricular filling pressure. Available data suggest that a pre-discharge clinical assessment of congestion is often not performed, and even when it is performed, it is not done systematically because no method to assess congestion prior to discharge has been validated. Grading congestion would be helpful for initiating and following response to therapy. We have reviewed a variety of strategies to assess congestion which should be considered in the care of patients admitted with HF. We propose a combination of available measurements of congestion. Key elements in the measurement of congestion include bedside assessment, laboratory analysis, and dynamic manoeuvres. These strategies expand by suggesting a routine assessment of congestion and a pre-discharge scoring system. A point system is used to quantify the degree of congestion. This score offers a new instrument to direct both current and investigational therapies designed to optimize volume status during and after hospitalization. In conclusion, this document reviews the available methods of evaluating congestion, provides suggestions on how to properly perform these measurements, and proposes a method to quantify the amount of congestion present.
Introduction
Acute heart failure (AHF) is defined as new-onset or worsening HF signs and symptoms requiring urgent therapy, most commonly in the hospital setting.1–3 Signs and symptoms usually improve markedly during hospitalization, however mortality during admission remains high, ranging from 5 to 15% or more.2,4–8 Many patients are managed by non-specialists and most patients have one or more serious co-morbid conditions that contribute to poor outcome and exclude patients from participation in clinical trials. Of those patients who survive to discharge, a further 10–15% will die within 6–12 weeks and about one-third will be re-admitted for a variety of reasons; often HF.2,4,5
Available data suggest that the main reason for hospitalization for worsening HF is related to the symptoms (dyspnoea or breathlessness) of congestion, manifested also by signs [e.g. jugular venous distension (JVD), rales, and oedema] of congestion, rather than low cardiac output.4,5,9–11 Although congestion is the main reason for hospitalization, many patients are discharged without losing body weight and with persistent signs of congestion.10,12 This may be a particular problem in US hospitals where admissions are much shorter than in Europe. Congestion is associated with a poor prognosis and is an important target for therapy.13,14
To the best of our knowledge, no systematic method to assess congestion prior to discharge has been proposed by available guidelines or research studies.15–17 This document reviews the available methods of evaluating congestion, provides suggestions on how to properly perform these measurements, and proposes a method to quantify the amount of congestion present.
Congestion in acute heart failure
Pathophysiology
Clinical congestion in HF is defined as a high left ventricular diastolic pressure (LVDP) associated with signs and symptoms of HF such as dyspnoea, rales, and oedema. Elevation of LVDP in HF patients without overt clinical congestion has been termed ‘haemodynamic congestion’.12 Often, haemodynamic congestion precedes clinical congestion by days or even weeks.18–22 Thus, clinical congestion may be the ‘tip of the iceberg’ of the haemodynamic derangements that precede symptoms (Figure Figure 1). In fact, in chronic HF, even severe haemodynamic congestion rarely causes rales and/or radiographic pulmonary oedema.23,24 This may be related to several adaptive pathophysiological changes such as increases in alveolar capillary membrane thickness, increased lymphatic drainage, and/or pulmonary hypertension.
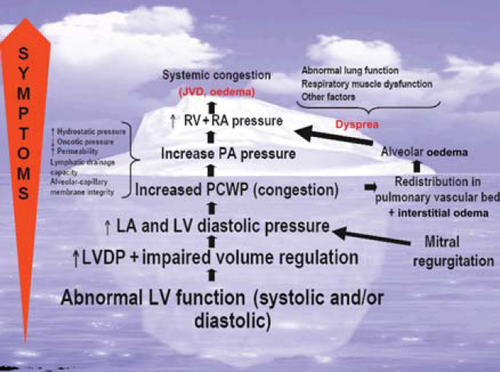
Theoretically, haemodynamic congestion may contribute to the progression of HF by further activating neurohormones and by causing subendocardial ischaemia, resulting in myocardial necrosis/apoptosis and/or secondary mitral insufficiency by its effects on LV geometry (changing it from an ellipsoid to a sphere).25–28 In addition, elevated right atrial pressure may contribute to the cardio-renal syndrome through reduction of the perfusion gradient across the kidneys.29–32
Prognosis
Clinical congestion has prognostic importance in HF patients. In a retrospective analysis of the Acute and Chronic Therapeutic Impact of a Vasopressin-2 Antagonist in Congestive Heart Failure (ACTIV in CHF) trial, patients with dyspnoea, oedema, and JVD on admission had a two- to three-fold increase in 60-day mortality compared with those without these features.33 Retrospective analysis of the Studies of Left Ventricular Dysfunction (SOLVD) treatment trial demonstrated that signs of congestion in patients with chronic HF were associated with 15 and 43% relative increases in the risk of all-cause death and HF hospitalization, respectively, during the mean follow-up period of 32 months.13 In a cohort of 146 decompensated HF patients, those with 0, 1–2, and ≥3 residual symptoms or signs of congestion (e.g. orthopnoea, JVD, oedema, weight gain, and new increase in baseline diuretics) had 2-year survival rates of 87, 67, and 41%, respectively.14 When patients admitted with HF were characterized according to their haemodynamic profile, those with evidence of congestion had higher rates of death or death plus urgent transplantation than other clinical profiles.34 The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) study found elevated pulmonary capillary wedge pressure (PCWP) and reduced 6 min walk to be the strongest independent predictors in post-discharge mortality after hospitalization for worsened HF among all clinical, haemodynamic, and laboratory variables studied.35 Several other studies have also shown that patients with haemodynamic congestion have poor outcomes.36–38
Therapy
Since congestion is also related to water and sodium retention, the main goal of therapy is to remove excess intravascular and extravascular fluid without further activation of neurohormones and without worsening renal function. In addition, these therapies should not cause myocardial damage. At present, the most widely used and relatively effective therapies for fluid removal are non-potassium sparing diuretics. Their use, however, has been associated with significant side effects (i.e. hypokalaemia, activation of neurohormones, arterial vasoconstriction, and worsening renal function).39–42 Worsening renal function occurs in 34% of hospitalized patients and is associated with poor prognosis.43 Ultrafiltration appears to remove fluid and reduce hospitalizations; however, this fluid removal may or may not be associated with improved survival.44 Vasopressin antagonists remove electrolyte-free water by blocking vasopressin receptors in the distal nephron. Analysis of the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) trial, which studied the effects of tolvaptan vs. placebo in patients hospitalized with worsening HF and LV ejection fraction (LVEF) ≤40%, demonstrated a significant reduction in symptoms of congestion due to loss in body fluid with tolvaptan; however, mortality and hospitalizations were unaffected when compared with placebo.45,46 Moreover, a drug that had a favourable effect on renal function (rolofylline, an adenosine antagonist) did not have a beneficial long-term effect in AHF.47,48 It is possible that the best way to keep off excess fluid to avoid decompensation is to improve overall cardiac function using evidence-based therapies such as beta-blockers, angiotensin-converting enzyme inhibitors, aldosterone receptor antagonists, and cardiac resynchronization therapy, although diuretics may still be necessary to maintain relief from congestion despite these measures.49
Assessment of congestion
Available guidelines recommend treatment to improve symptoms and signs of congestion and that patients achieve near-optimal volume status prior to discharge; however, there is no established algorithm for the assessment of congestion.16 Currently, the gold standard for evaluating haemodynamic congestion in HF patients is cardiac catheterization to measure right atrial pressure and PCWP.50 However, the invasive nature of catheterization limits its routine use in practice. No single non-invasive test can accurately detect haemodynamic congestion, and the ability to detect congestion by haemodynamic measurements remains a diagnostic challenge because it usually precedes clinical symptoms. Relying on a limited set of physical examination findings and/or radiographic signs has met with low sensitivity and poor predictive value. In a series of 50 patients with chronic HF, physical signs of congestion [rales, oedema, and elevated jugular venous pressure (JVP)] were absent in 42% of the patients with a measured PCWP ≥22 mmHg and the combination of these signs showed only a 58% sensitivity in detecting elevated PCWP.24 In another study of 52 patients with chronic HF referred for evaluation for heart transplantation, physical signs (orthopnoea, oedema, rales, third heart sound, and elevated JVP) or radiographic signs (cardiomegaly, vascular redistribution, and interstitial and alveolar oedema) had poor predictive value for identifying patients with elevated PCWP.51 In another study of 52 chronic HF patients, the presence of JVD, when measured carefully, had the best combination of sensitivity (81%), specificity (80%), and predictive accuracy (81%) for detection of an elevation in PCWP (≥18 mmHg).52 In the ESCAPE trial, JVD and orthopnoea were the only two findings from history and physical examination associated with an elevated PCWP.53
Proposed pre-discharge assessment of congestion
There are several ways of assessing congestion, which have been evaluated individually. Each measurement in isolation has diagnostic limitations in sensitivity, specificity, and predictive value (Table Table 1). However, no single study has assessed all of these measurements simultaneously. A summary of the advantages and limitations of different methods used for measuring congestion is presented in Table Table 2.
| Sign or symptom | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Dyspnoea on exertion | 66 | 52 | 45 | 27 |
| Orthopnoea | 66 | 47 | 61 | 37 |
| Oedema | 46 | 73 | 79 | 46 |
| Resting JVD | 70 | 79 | 85 | 62 |
| S3 | 73 | 42 | 66 | 44 |
| Chest X-ray | ||||
| Cardiomegaly | 97 | 10 | 61 | — |
| Redistribution | 60 | 68 | 75 | 52 |
| Interstitial oedema | 60 | 73 | 78 | 53 |
| Pleural effusion | 43 | 79 | 76 | 47 |
| Measurement | Advantages | Limitations |
|---|---|---|
| Resting dyspnoea and orthopnoea | Rapid assessment | May be non-cardiac in origin |
| Dyspnoea on exertion | Provides functional information | May be non-cardiac in origin |
| Rales | Rapid assessment | Not sensitive or specific for congestion |
| Jugular venous pressure | Good sensitivity and specificity | Difficult to assess in obesity; intraobserver variation |
| Oedema | Simple measurement | May not represent congestion; must correlate with jugular venous pressure |
| Body weight | Simple measurement | Fluctuations may not represent changes in intravascular volume |
| Serum sodium | Predicts outcomes | |
| Urea nitrogen | Predicts outcomes | |
| Natriuretic peptides | Predict outcomes | Do not change acutely (B-type peptides); elevations found in other conditions such as renal disease or cirrhosis |
| Radiographic congestion | Not sensitive or specific | |
| Orthostatic testing | Important guide for therapy | Complex measurement |
| Valsalva manoeuvre | Dependent on patient effort; can require specialized equipment | |
| Sublingual nitroglycerin | Impractical in most patients | |
| Portable ultrasound | Evolving technology | Requires training and specialized equipment |
Bedside assessment
Breathlessness/dyspnoea/orthopnoea
Given the dominance of dyspnoea as a presenting symptom, its relief or improvement is an important marker of reduced congestion. At the present time, however, there is no validated method of assessing breathlessness/dyspnoea in HF patients. In the meantime, a consensus proposal has been put forward to facilitate standardization of measurement.54 Both a Likert scale and/or visual analogue scale may be used to measure dyspnoea. The Likert and the visual analogue scores are initially measured in the sitting position (60°) and then, if the patient is not already too breathless, in the supine position (20°) after at least 2 min if tolerated. Symptoms should be measured on an absolute scale (e.g. I am very breathless or not at all breathless) rather than a relative scale (e.g. do not ask about whether symptoms have improved) since the latter may be more difficult to interpret as it requires patients to remember their symptomatic state at the time of presentation.
Paroxysmal nocturnal dyspnoea is a symptom that occurs during sleep, causes the patient to wake up with severe breathlessness, and is relieved by sitting upright. Paroxysmal nocturnal dyspnoea is an important symptom which often precedes pulmonary oedema by several nights/days. Similar symptoms can occur in chronic obstructive pulmonary disease.
Orthopnoea has been shown to correlate with high PCWP with a sensitivity approaching 90%.34 Persistent orthopnoea was associated with a higher rate of hospitalization when patients were followed up for 6 months after discharge.55 Orthopnoea can be tested by asking the patient to lie supine for a defined period of time (e.g. 2 min) while monitoring respiratory rate and breathlessness. Supine positioning results in the mobilization of fluid from dependent venous reservoirs in the abdomen and the lower extremities, which increases venous return (∼250–500 cc fluid) to the thoracic compartment. As a result, pulmonary venous and capillary pressures rise further, elevating already high right- and left-sided filling pressures. This may result in interstitial pulmonary oedema, reduced pulmonary compliance, increased airway resistance, and dyspnoea. At the time of discharge, excluding those with advanced/end-stage HF, patients should be able to lie supine or with no more than one pillow (unless more pillows are necessary for other medical conditions such as back pain or gastro-oesophageal reflux) without developing breathlessness.
Dyspnoea and orthopnoea are symptoms that may or may not be cardiac in origin. The severity of dyspnoea or orthopnoea may reflect the rapidity of the rise in PCWP as well as its absolute value. Further research in this area is needed, given the dominance of breathlessness as a presenting complaint.54
Dyspnoea on exertion
Functional capacity has been shown to predict mortality and hospitalizations in HF patients.56 As might be expected, dyspnoea on exertion is the most common persisting symptom at discharge.10 Ideally, patients without locomotor problems should be able to walk for at least 6 min on level ground without undue breathlessness and complete at least 200 m, without postural symptoms of dizziness or light-headedness.
Rales or crepitations
Auscultation of rales may indicate fluid overload but is non-specific. The absence of rales is not a sensitive marker of the absence of congestion.51 Rales should be examined after the patient has been asked to cough. If rales persist at the time of discharge, it should be noted whether they are due to other conditions such as pneumonia, interstitial fibrosis, chronic bronchitis, asthma, or emphysema or whether persistent congestion due to HF remains.
Jugular venous pressure
Jugular venous pressure reflects right atrial pressure, which usually indicates an elevated PCWP in patients with HF.57 When performed properly and by experienced physicians, JVP estimation is fairly accurate.53,58 Strategies to improve measurement have been suggested.58,59 In some individuals with pulmonary hypertension or tricuspid regurgitation, a high JVP is required to maintain adequate left-sided filling pressures, and normalizing JVP prior to discharge is not in the patient's interest.60 The hepato-jugular reflux may also be used to assess elevated pressure and is both sensitive and reliable.61,62 Measurement of JVP is often limited by patient body habitus such as obesity or respiratory pathology. Although there are limitations, JVP elevation is associated with an increased risk for HF hospitalization, is a simple measurement of congestion, and is thus a potential target to monitor therapy.13 However, its use in multi-centre studies is not reliable without adequate audit of clinical expertise in each centre.
Oedema
In HF patients, peripheral oedema is usually associated with a high right atrial pressure that is most commonly due to left-sided HF. Lower extremity oedema frequently improves or resolves with diuretic therapy during hospitalization. Oedema may also redistribute to dependent areas during hospital bed rest (i.e. sacral oedema) and is commonly missed by a casual examination. Apparent improvement without weight loss during hospitalization suggests redistribution of fluids. Therefore, both the sacrum and lower limbs should be examined and weight charts reviewed prior to discharge.
A major limitation in using oedema as a measurement of congestion is that it may not be related to high filling pressures, but rather reflect extravascular volume shifts resulting from low plasma oncotic pressure, high vascular permeability, or both.24 An elevated JVP improves the specificity of oedema as a sign of congestion. Recognizing these limitations, at the time of discharge, patients should have no more than trace oedema unless they have pre-existing oedema of non-cardiac aetiology (e.g. liver cirrhosis, venous insufficiency, renal failure, and hypoalbuminaemia).
Body weight
Congestion as a result of sodium and water retention in HF patients is often manifested by an increase in body weight. Although a change in body weight observed over time (weeks or months) may be associated with other contributing factors such as malnutrition and deconditioning (cardiac cachexia), daily variation most likely reflects changes in volume status. Thus, an acute change in body weight is a reasonable marker of fluid balance. Measurement of body weight should be performed as consistently as possible (using a standardized scale, preferably with a precision of 50 g, at the same time of the day, post-void, prior to eating, prior to the medication dose, and with patients wearing the same clothing). The scales should stand on a flat, solid surface rather than carpets unless specifically designed for use in that setting.
One limitation to body weight measurement is that fluctuations may not always reflect changes in intravascular volume. For example, in the EVEREST study, patients hospitalized for worsened HF who were receiving the selective vasopressin-2 receptor antagonist tolvaptan had reduced body weight compared with those receiving placebo, but there was no significant effect on peripheral oedema45 Another difficulty with using body weight as a measure of congestion is that the degree of absolute weight loss may not be as important in patients with acute hypertensive HF because some of these patients may be euvolaemic with pulmonary but not systemic congestion as a result of volume redistribution.,11,52 Absolute weight loss is more important in patients with profound anasarca, who require more fluid removal than patients without. Ideally, patients should be at ‘target’ or at least at lower body weight compared with admission. However, adequate diuresis may be prevented by worsening renal function and/or hypotension.
Laboratory and radiographic assessment
Blood urea nitrogen
Fluid balance in HF involves a complex interaction between the heart and the kidneys.32 In response to reduced cardiac output, there is a release of neurohormones by the sympathetic nervous system, renin–angiotensin–aldosterone system, and the arginine vasopressin system. This promotes fluid retention by the kidneys, as well as renal and systemic vasoconstriction. Renal dysfunction, as measured by serum creatinine or estimated glomerular filtration rate (GFR), consistently predicts poor outcomes in HF in the chronic63,64 and acute65–68 settings.
Blood urea nitrogen (BUN in the USA and urea in Europe) has been investigated as a potential prognostic marker [urea (in mmol/L) = BUN (in mg/dL of nitrogen)/2.8].
Urea is produced in the liver as the degradation product of proteins and is filtered by the kidneys. Unlike creatinine, which is actively secreted and not reabsorbed, ∼40–50% of the filtered urea is reabsorbed, predominantly in the proximal tubule, paralleling reabsorption of sodium and water. Increases in urea in HF may reflect congestion and fluid retention, as well as cardiac and renal dysfunction, whereas elevation in creatinine is more specific for changes in GFR.69 Recently, BUN was found to be a better predictor of outcome than creatinine or estimated GFR in AHF.70–73 Elevations in BUN disproportionate to the rise in creatinine (20:1 for BUN vs. creatinine in mg/dL or serum urea >10% of the value of serum creatinine in mmol/L) may also reflect dehydration.
Natriuretic peptides
Natriuretic peptides [NPs; brain natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP)] are neurohormones specifically secreted from the cardiac chambers in response to volume and pressure overload leading to increased wall tension. High pre-discharge NP values are strong, independent predictors of death or re-admission after hospitalization for AHF, and incremental to other clinical and laboratory variables.74,75 Thus, measurement of NPs prior to discharge can be useful in identifying patients at high risk for adverse events. A decrease in initially high NPs observed in patients with congestion before discharge may be a useful indicator of a reduction in filling pressure.76 However, the utility of NP levels may be limited by the fact that their production and release may lag behind acute changes in haemodynamic measurements.77 Increases in NP levels are non-specific and may reflect cardiac stress including sepsis, pulmonary disease, and renal dysfunction even when no important cardiac disease is present. In this setting, they may be markers of cardiac stress rather than cardiac disease. This may be why they are better as markers of prognosis than accurate diagnostic tools. Importantly, NPs cannot be used alone to assess congestion but must be evaluated as with most tests in the appropriate clinical context. A patient with high NPs at discharge (NT-proBNP > 1500 pg/mL and BNP > 300 pg/mL) should be considered at high risk of death or re-admission. However, the increase in risk as NP levels rise is linear and use of a single, precise cut-point is not appropriate. Values are best used to describe low, medium, and high risk. Adequately sized studies do not yet exist to provide more precise direction.
Chest radiography
The absence of chest X-ray findings of HF (e.g. cardiomegaly, vascular redistribution, and interstitial and alveolar oedema) does not exclude a high PCWP. Radiographic signs of pulmonary congestion are absent in 53% of the patients with PCWP of 16–29 mmHg and in 39% of the patients with PCWP of ≥30 mmHg.51
Dynamic manoeuvres
Orthostasis
The normal haemodynamic response in patients with normal LV function and filling pressures when their position is changed from sitting to standing is a slight reduction in blood pressure (by 4 mmHg systolic and 5 mmHg diastolic) and an increase in heart rate.78 In these patients, significant volume depletion or blood loss results in a reduction in preload and cardiac output, manifesting in large changes in blood pressure (greater than equal to −20 mmHg systolic) and heart rate (≥10–30 b.p.m.), depending on baseline conditions and the use of beta-blocking agents. However, in many patients with HF and high filling pressures, orthostatic postural changes may result in improved haemodynamics, potentially leading to relief of subendocardial ischaemia, attenuation of sphericity, and mitral regurgitation, and reductions in preload leading to an increase in cardiac output.25 As a result, these patients may actually have an increase in systolic blood pressure with orthostatic changes. When these patients have lower filling pressures, the orthostatic changes will not change their cardiac output and not have the paradoxical postural increase in blood pressure.79 Therefore, in patients with HF with an initial paradoxical increase in blood pressure with orthostasis, documentation of lack of a blood pressure increase may indicate a euvolaemic state. This observation cannot be applied to patients with other forms of cardiomyopathy, such as hypertrophic cardiomyopathy or aortic stenosis, where normal cardiac output is dependent on elevated filling pressures. In addition, blood pressure cannot be measured reliably in patients with atrial fibrillation using standard instruments.
When measuring postural vital signs, clinicians should wait at least 2 min before performing measurements in the supine position, and at least 1 min before measuring vital signs in the upright position in order to allow the postural haemodynamic changes to take effect. Care should be taken to use a properly sized blood pressure cuff and to keep the cuff at the level of the heart.
A major limitation to the use of orthostasis for determining the presence of congestion is that one must know whether the HF patient has a dilated ventricle or other forms of systolic or diastolic failure. Nevertheless, orthostatic changes are of practical importance because they provide a benchmark against which one may measure volume status and diuretic response.
Valsalva manoeuvre
Although generally underutilized in clinical practice, assessment of blood pressure in response to the Valsalva manoeuvre may have a role as a non-invasive bedside tool to evaluate volume status. Bedside sphygmomanometry can provide a qualitative assessment of volume status, and commercially available arterial waveform devices provide a rapid and reliable beat-to-beat measurement of cardiovascular dynamics that can estimate LV filling pressures. Studies have demonstrated a high correlation between the cardiovascular response to the Valsalva manoeuvre and invasively measured ventricular filling pressures in HF patients.80,81 The normal blood pressure response to the Valsalva manoeuvre is an initial increase with the onset of straining caused by an acute increase in intrathoracic pressure (Phase 1), followed by a sharp decrease to below baseline levels as the straining is maintained caused by a decreased venous return and compensatory increase in systemic vascular resistance and heart rate (Phase 2).82 After the strain is released, there is a short reduction in arterial pressure due to an acute reduction in intrathoracic pressure (Phase 3), followed by an overshoot in arterial pressure due to increased venous return with a decrease in systemic vascular resistance and heart rate (Phase 4). In patients with mild HF, Phases 1 through 3 are normal, but Phase 4 is absent (‘absent overshoot’ pattern). In patients with advanced HF, after Phase 1, the increase in blood pressure remains elevated during the entire strain period, returning to baseline after release in Phase 3 (‘square wave’ pattern). The mechanism behind this abnormal response is the maintenance of LV filling from increased central blood volume that maintains stroke volume, despite changes in intrathoracic pressures.80,83
Bedside sphygmomanometry can assess normal and abnormal responses to the Valsalva manoeuvre, as originally described in 1956.84 After the cuff is inflated and maintained at 15 mmHg higher than systolic blood pressure, the Valsalva manoeuvre is performed while Korotkoff sounds are auscultated over the brachial artery. The normal response is when only Phases 1 and 4 are registered, ‘absent overshoot’ is when only Phase 1 is registered, and the ‘square wave’ is when only Phases 1 and 2 are registered.85 This method has been shown to be clinically useful in ambulatory patients; however, it is dependent on patient effort.86
Future directions
Echocardiography and portable ultrasound
Echocardiography is the most commonly used non-invasive tool to assess cardiovascular structure and function but may be misleading in AHF. Compared with NPs, LVEF is a poor predictor of prognosis; however, other echocardiographic parameters may be used to estimate the severity of congestion.87 Right and left atrial pressures can also be estimated by echocardiography in expert hands, although with less certainty in AHF.70–74 Estimation of right atrial pressure can be performed using any two-dimensional echocardiography platform by measuring the diameter of the inferior vena cava (IVC).88 In a healthy state, IVC diameter collapses upon inspiration and expands during expiration due to the changes in intrathoracic pressure with respiration. High right atrial pressures dilate the IVC and blunt this normal IVC collapsibility. Therefore, small, collapsible IVCs as visualized by echocardiography represent low right atrial pressures, whereas large, non-collapsible IVCs reflect high right atrial pressures. A dilated, non-collapsible IVC was one of a few predictors of readmission after hospitalization for HF in a small observational study.89
Left atrial pressure can be estimated by observing diastolic events using Doppler and tissue Doppler imaging. One of the earliest events in diastolic and systolic dysfunction is the reduction in myocardial velocity in early diastole and can be measured by the E′-wave using tissue Doppler imaging. As filling pressures increase, early mitral inflow velocities, as measured by the E-wave using pulse-wave Doppler, also increase. The ratio of E/E′ has been found to correlate well with PCWP.90–92 In addition, an elevated E/E′ ratio has been shown to predict adverse outcomes in HF in small series of patients in expert hands and is complementary to such measurements as BNP and expert clinical assessment.93–95 However, patients with mitral regurgitation were excluded from these early studies, and in recent studies, the correlation in this patient subset may be less robust than previously reported.96
Ultrasonography of the lungs using an echocardiographic probe is another potentially useful way to assess pulmonary congestion. In patients with pulmonary congestion, images defined as ‘ultrasound lung comets’ can be visualized by scanning with cardiac probes along the intercostal spaces.97 A correlation exists between the number of ‘ultrasound lung comets,’ pulmonary congestion demonstrated by radiographic signs, interstitial oedema documented by computed tomography, extravascular lung water measured by the indicator dilution technique, and PCWP.98,99
Although echocardiography is a simple method of estimating EF and filling pressures, it is not practical to perform and repeat these measurements on every patient admitted with HF. Portable ultrasound may have promise in routine and serial assessment of HF patients.100
Thoracic impedance monitoring
Thoracic impedance measurement provided by an external or implanted device has been investigated in recent years.95 Several studies have shown that decreasing thoracic impedance correlates with HF hospitalizations.19,101,102 There are studies ongoing to determine the clinical effectiveness of outpatient monitoring of thoracic impedance with the hope of decreasing HF hospitalizations and improving outcomes. Should these techniques prove effective, many patients could be evaluated prior to hospital discharge to confirm euvolaemia.103
Blood pool analysis
Another method of assessing volume status is blood volume analysis. This technique uses radioisotopes tagged to red cells or albumin to directly measure blood volume. It correlates with invasive measurements of cardiac filling pressures in patients presenting with decompensated HF.104–108 This technique has existed for quite some time, but more clinical trials are needed before it is used to use this to guide therapy.109
Grading congestion
The above measurements each have their strengths and weaknesses. Some measurements are only predictive of congestion in the context of other congestion findings. Similarly, some measurements predict congestion from right-sided HF, whereas others predict congestion from left-sided HF or biventricular failure.
A systematic approach to grading congestion would be helpful in initiating and following response to therapy. Previous scores have used only clinical examination to evaluate congestion.34,110 We propose that a combination of available measurements of congestion be prospectively tested for their ability to predict re-hospitalization after admission for AHF. Once this is established, therapy for fluid removal could be adjusted with the goal of reducing the overall amount of congestion, with re-hospitalization as the measured outcome (if the treatment is safe). Key elements in the measurement of congestion include bedside assessment, laboratory analysis, and dynamic manoeuvres, as outlined above and summarized in Table Table 3. A point system is used to quantify the degree of congestion. We anticipate that this model will not be the one finally selected for regular clinical use, but it provides a crucial starting point from which to evolve. Ultimately, a revision of this score offers a new instrument to direct both current and investigational therapies designed to optimize volume status during and after hospitalization.
| Variable | Score | ||||
|---|---|---|---|---|---|
| −1 | 0 | 1 | 2 | 3 | |
| Bedside assessment | |||||
| Orthopnoeaa | None | Mild | Moderate | Severe/worst | |
| JVP (cm) | <8 and no hepatojugular reflux | 8–10 or hepatojugular reflux | 11–15 | >16 | |
| Hepatomegaly | Absent in the setting of normal JVP | Absent | Liver edge | Moderate pulsatile enlargement | Massive tender enlargement extending to midline |
| Oedema | None | 1+ | 2+ | 3+/4+ | |
| Laboratory | |||||
| Natriuretic peptides (one) | |||||
| BNP | <100 | 100–299 | 300–500 | >500 | |
| NT pro-BNP | <400 | 400–1500 | 1500–3000 | >3000 | |
| Dynamic manoeuvres | |||||
| Orthostatic testing | Significant decrease in SBP or increase in HR | No change in SBP or HR | |||
| No difficulty | Mild | Moderate | Severe/worst | ||
| 6 min walk test | >400 m | 300–400 m | 200–300 m | 100–200 m | <100 m |
| Valsalva manoeuvre | Normal response | Absent overshoot pattern | Square wave pattern | ||
- a Congestion grade: <1, none; 1–7, mild; 8–14, moderate; 15–20, severe. Oedema, in the absence of other cause of oedema.
- a Orthopnoea: 0, absent; mild (use of one pillow); moderate (use of more than one pillow); severe, sleeps in an armchair on in a seated position.
Summary
Although congestion, not a low cardiac output, is the main cause for hospitalization, many HF patients are discharged with persistent signs and symptoms of congestion and/or a high LV filling pressure. Available data suggest that a pre-discharge clinical assessment of congestion is often not performed, and even when it is performed, it is not done systematically. We have reviewed a variety of strategies to assess congestion which should be considered in the care of patients admitted with HF. These strategies expand upon guideline recommendations for optimizing volume status and inpatient HF performance measures, by suggesting a routine assessment of congestion and a pre-discharge scoring system.
Acknowledgements
The authors would like to thank the members of the Board of the Heart Failure Association of the European Society of Cardiology and the Committee on Acute Heart Failure of the Heart Failure Association of the European Society of Cardiology for useful comments on the manuscript. Board of the Heart Failure Association of the European Society of Cardiology: J.M., K.D., P.P., G.F., A. Shah, T.J., S.D.A., M. Cowie, U. Dahlström, I. Ekman, T. Eschenhagen, G. Hasenfuß, S. Janssens, A. Maggioni, Z. Papp, W. Paulus, B. Pieske, M. Ryder, P.S., D.J.V., F.Z., and K. Swedberg. Committee on Acute Heart Failure of the Heart Failure Association of the European Society of Cardiology: G.F., P.P., P.S., F.Z., D.L.B., P. de Groote, F.F., M.G., G.J., J.L.-S., A.M., M.M., M.N., A.R., and J.L. Vincent.
Conflict of interest: M.G. is a consultant for Abbott Labs, Astellas, AstraZeneca, Bayer Schering Pharma AG, CorThera Inc., Cytokinetics Inc., DebioPharm SA, Errekappa Terapeutici (Milan, Italy), GlaxoSmithKline, Ikaria, Johnson and Johnson, Medtronic, Merck, Novartis Pharma AG, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, Sanofi Aventis, Sigma Tau, Solvay Pharmaceuticals, and Trevena Therapeutics, and has received honoraria from Medtronic, Novartis Pharma AG, Otsuka Pharmaceuticals, Sigma Tau, Solvay Pharmaceuticals, DebioPharm SA, and Pericor Therapeutics. P.P. has received honoraria from Merck and Biogen. J.G.C. has conducted research in acute heart failure sponsored by Otsuka, MSD, Orion, Corthera, and Biogen. G.C.F. has received research grants from NIH, has received honoraria from AstraZeneca, GlaxoSmithKline, Medtronic, Merck, Novartis, and Pfizer and has consulted for GlaxoSmithKline, Medtronic, Merck, Novartis, St Jude, Pfizer, Sanofi, and Scios. A.M. is a consultant to Abbott, Orion Pharma, Bayer Pharma, and the Medicine Company and has received lecture fees from Abbott, Guidant, Inverness, and Edwards Life Sciences. M.M. has received occasional honoraria and travel reimbursement for participation in Advisory Board Meetings and symposia from Corthera, Cardiokine, Merck, and Otsuka. M.N. is a member of the study leadership. P.S.P. is a consultant for Astellas, Bayer, EKR Therapeutics, Johnson and Johnson, the Medicines Company, Otsuka, Palatain Technologies, PDL BioPharma, Pericor Therapeutics, and Solvay Pharmaceuticals, and has received honoraria from Biogen Idec Corthera, Ikaria, and Nile Therapeutics and research support from Merck and PDL BioPharma. L.W.S. has received modest consulting fees from Medtronic Inc. D.J.V. has received consulting fees from Medtronic and Biosite. F.Z. has received consulting honoraria from Servier, AstraZeneca, Pfizer, Boehringer Ingelheim, Novartis, Abbott, Relypsa, Resmed, Merck, Daiichi Sankyo, Takeda, Boston Scientific, Medtronic, and Otsuka. S.D.A. has been a consultant for Otsuka Pharma, Biogen Idec Merck Inc., and Solvay and has received honoraria for speaking from Otsuka Pharma. A.R. has received consulting and/or lecturing fees from Abbott, LidCO, and Cheetah.




