Serum levels of large tenascin-C variants, matrix metalloproteinase-9, and tissue inhibitors of matrix metalloproteinases in concentric versus eccentric left ventricular hypertrophy
Abstract
Aims
Chronic hypertension may cause left ventricular hypertrophy (LVH). The role of matrix metalloproteinases (MMPs), tissue inhibitors of matrix metalloproteinases (TIMPs), and tenascin-C (Tn-C) splice variants in concentric vs. eccentric left ventricular remodelling has not been investigated.
Methods and results
Serum levels of B or C domain containing Tn-C, MMP-9, TIMP-1, −2, and −4 were determined in concentric (left ventricular posterior wall thickness >13 mm and intraventricular septum >13 mm, n = 61) and eccentric (end-diastolic left ventricular diameter >55 mm or end-systolic left ventricular diameter >40 mm, n = 34) LVH by enzyme-linked immunoassays. Levels of B domain containing Tn-C were higher in patients with LVH than in normal volunteers (P = 0.020) and higher in eccentric LVH (EH) compared with concentric LVH (CH) (P = 0.003). A cut-off value of 900 ng/mL might discriminate between these different forms of LVH. Matrix metalloproteinase-9 was higher in patients with LVH than in normal volunteers (P = 0.042), and levels were decreased in EH compared with CH (P = 0.028). Patients with LVH had higher levels of TIMP-1 (P = 0.059), TIMP-2 (P = 0.043), and TIMP-4 (P = 0.163) than normal volunteers, but there were no differences between the LVH groups.
Conclusion
Our data suggest that myocardial remodelling in LVH is associated with changes in serum levels of MMP-9, TIMP-1, −2, −4, and Tn-C splice variants. In addition, B domain containing Tn-C discriminated EH from CH and might be suggested as a potential diagnostic marker.
Introduction
Arterial hypertension is known to cause left ventricular hypertrophy (LVH) accompanied by changes in cardiac function.1,2 Left ventricular hypertrophy occurs as pressure overload hypertrophy (POH) or volume overload hypertrophy (VOH). Pressure overload hypertrophy is characterized by a concentric hypertrophy with increased wall thickness and unaltered or decreased LV diameter. Volume overload hypertrophy shows an eccentric hypertrophy with increased LV diameter and unaltered or decreased wall thickness.3,4 Concentric hypertrophy is known as the classic paradigm of human LV compensation in response to chronic hypertension.5 However, it has been shown that hypertensive patients can also develop eccentric LVH (EH).6,7 Both LV mass and LV geometry are of high clinical interest since both represent risk factors in hypertensive patients for major events like stroke, myocardial infarction, or cardiovascular death, independent of blood pressure.4,8–10 The question which hypertensive patient will develop concentric LVH (CH) or EH has not been answered until now. A variety of conditions such as total peripheral resistance and reduced or elevated stroke volume and arterial stiffness4 are known to influence the development of LVH. However, prediction of their clinical manifestations in the early stages of arterial hypertension is not possible. Tissue remodelling in LVH is accompanied by structural and functional changes in the myocardial extracellular matrix (ECM) mediated by alterations in the ratio of matrix synthesis and degradation3,11–13 Moreover, the re-expression of oncofoetal variants of the adhesion proteins fibronectin, laminin, and tenascin-C (Tn-C) have been shown to be of functional importance.14–16
Tenascin-C is an ECM glycoprotein which occurs as different variants generated by alternative splicing. Large variants are known to be crucial for the development of the myocardium, heart valves, and coronary vessels in the early stages of embryogenesis17 but are not detectable in adults. Under pathological conditions associated with tissue remodelling such as myocardial injury or acute myocardial infarction, Tn-C is re-expressed.14,18–21 In patients with dilated cardiomyopathy and chronic heart failure, Tn-C levels correlate positively with NYHA class and LV diameters and negatively with left ventricular ejection fraction (LVEF).22,23 To our knowledge, differential expression of Tn-C splicing variants in hypertensive heart disease has not been investigated until now. Oncofoetal splice variants of Tn-C, especially the large isoforms, show a higher susceptibility to degradation by matrix metalloproteinases (MMPs).24 Thus, a functional interaction of the large splice variants of Tn-C with special MMP subtypes under pathological conditions may be important in the molecular pathogenesis of LVH.
Matrix metalloproteinases as a family of zinc-dependent enzymes and their tissue inhibitors (TIMPs) play an important role in myocardial matrix remodelling.25,26 In POH, there are increased serum levels of TIMPs in the early stages of progression reflecting matrix accumulation and fibrosis; increased serum levels of MMPs in the later stages are associated with matrix degradation, loss of structural integrity, and declining LV function.27–29 In animal models of VOH, increased myocardial MMP levels could be demonstrated even in the early stages of disease, which contributed to dilatation of the LV.3,30 Also, during ECM remodelling secondary to chronic hypertension, the myocardial MMP/TIMP ratio plays an essential role.13 In patients with heart failure, MMP-9 has been suggested to be a useful serum marker to monitor myocardial matrix remodelling and thus for providing prognostic information.31
Our study aimed to analyse the serum concentrations of B or C domain containing splice variants of Tn-C, the serum concentrations of MMP-9, and the tissue inhibitors of matrix metalloproteinases (TIMP)-1, −2, and −4 in patients with CH and EH. Serum markers showing significant differences between both forms of LVH could be used to provide new insights into the role of these molecules in LVH-associated myocardial remodelling and to define possible new serum markers for the differential diagnosis of EH vs. CH.
Methods
Study population
A total of 95 patients with a history of arterial hypertension and echocardiographic signs of hypertensive heart disease were included. Twelve normal volunteers (six men, six women; mean age 57 ± 12 years) were also evaluated. Patients were admitted to the University Hospital of Jena, Clinic of Internal Medicine I, Germany, between January 2006 and June 2008. Age, gender, blood pressure, heart rate, and echocardiographic signs of hypertensive heart disease were evaluated. Written informed consent was given by all patients and volunteers. The study was approved by the local Ethics Committee of the Friedrich Schiller University of Jena, Germany.
The study inclusion criteria focused on patient's data that were clearly available and comprehensive. All patients were required to have a history of arterial hypertension for at least 5 years. Patients were divided into two groups according to echocardiographic parameters. The first group comprised 61 patients with CH, these patients had a left ventricular posterior wall thickness of >13 mm and an intraventricular septum in diastole of >13 mm [end-diastolic left ventricular diameter (LVDd) of <55 mm and end-systolic left ventricular diameter (LVDs) of <40 mm]. The second group comprised 34 patients with an EH, these patients had an LVDd of >55 mm and an LVDs of >40 mm.32 Left ventricular hypertrophy was due to arterial hypertension in all cases. Patients with other aetiologies, e.g. aortic stenosis as the cause of CH or ischaemic cardiomyopathy due to coronary heart disease as the cause of EH, were excluded. In patients with EH, coronary artery disease was excluded by coronary angiography. In order to minimize the impact of non-cardiac sources of ECM components or proteolytic enzymes on measured serum values, all patients with any diseases known to be associated with ECM remodelling such as acute or chronic inflammation, neoplastic diseases, chronic obstructive pulmonary disease (COPD) or other organ fibroses were excluded.
Assessment of serum levels of tenascin-C, matrix metalloproteinases, and tissue inhibitors of matrix metalloproteinases
Blood samples were collected and centrifuged at 2383 g for 25 min within 30 min of collection. Supernatants were stored at −20°C until analysis. Repeated freeze–thaw cycles were avoided.
Serum levels of B and C domains containing Tn-C were quantified using the Tenascin-C Large (FNIIIB) ELISA kit {IBL, Hamburg, Germany; sensitivity: 44 pg/mL; intra- and interassay precision [coefficient of variation (CV) values] were ≤10 and ≤9.4%} and the Tenascin-C Large (FNIIIC) ELISA kit [IBL; sensitivity: 0.01 ng/mL; intra- and interassay precision (CV values) were ≤6.6 and ≤7%] according to the manufacturer's instructions. For analysis, serum was pre-diluted in PBS 1:800 and 1:8, respectively. Serum levels of MMP-9 were quantified using the Quantikine® Human MMP-9 (total) Immunoassay [R&D Systems, Minneapolis, MN, USA; sensitivity: 0.156 ng/mL; intra- and interassay precision (CV values) were ≤3 and ≤8%] according to the manufacturer's instructions (serum pre-dilution, 1:100). Serum levels of TIMP-1, −2, and −4 were quantified using the Quantikine® Human TIMP-1 Immunoassay [sensitivity: 0.08 ng/mL; intra- and interassay precision (CV values) were ≤5 and ≤4.9%], the Quantikine® Human TIMP-2 Immunoassay [sensitivity: ranged from 0.004 to 0.064 ng/mL; intra- and interassay precision (CV values) were ≤4.4 and ≤7.3%], and the Quantikine® Human TIMP-4 Immunoassay [sensitivity: ranged from 2.14 to 10.0 pg/mL; intra- and interassay precision (CV values) were ≤5.6 and ≤9.2%] (R&D Systems) according to the instructions of the manufacturer (serum pre-dilutions: TIMP-1, 1:100; TIMP-2, 1:50; TIMP-4, not diluted). Absorbance at 450 nm was determined using an ELISA plate reader (LAMBDA KC4 Reader, MWG-BIOTECH AG). Serum protein levels were calculated on the basis of standard curves.
Statistical analysis
Data analysis was performed using SPSS software 15.0 (Microsoft). Data from the ELISA results were subjected to an analysis of variance (ANOVA) to determine differences in the continuous variables between the investigated groups. A P-value ≤0.05 indicated statistical significance. For B domain containing Tn-C, a receiver operating characteristic (ROC) curve was generated to elucidate a possible cut-off value which might discriminate between CH and EH.
Results
The clinical characteristics of the 95 patients included in the study are shown in Table 1.
| Form of LVH | Concentric LVH (n = 61) | Eccentric LVH (n = 34) |
|---|---|---|
| Age (years) | 68 ± 11 | 63 ± 13 |
| Men | 44 | 23 |
| Women | 17 | 11 |
| Heart rate (b.p.m.) | 70 ± 10 | 74 ± 11 |
| Systolic blood pressure (mmHg) | 133 ± 23 | 134 ± 23 |
| Diastolic blood pressure (mmHg) | 78 ± 13 | 78 ± 11 |
| Left ventricular posterior wall thickness (mm) | 13.51 ± 1.73 | 11.71 ± 1.8 |
| Intraventricular septum in diastole (mm) | 14.26 ± 1.75 | 11.59 ± 1.99 |
| End-diastolic left ventricular diameter (mm) | 48.37 ± 5.89 | 60.41 ± 8.66 |
| End-systolic left ventricular diameter (mm) | 31 ± 7.64 | 45.97 ± 8.83 |
- a Data are given as means ± SD or number (%).
Differences in serum levels of B and C domains containing tenascin-C in concentric vs. eccentric left ventricular hypertrophy
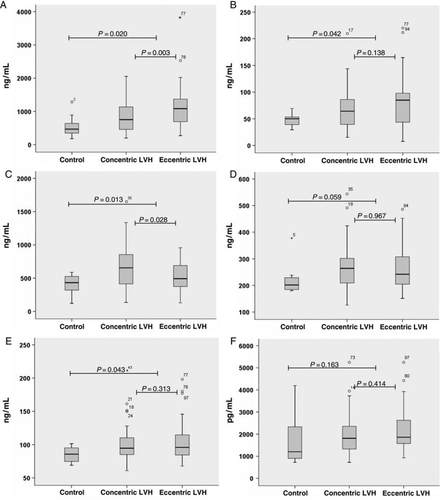
For B domain containing Tn-C, statistically significant differences in the serum concentrations between the three groups (normal volunteers, patients with CH, and patients with EH) were detected (P = 0.001) (Figure 1A). For C domain containing Tn-C, a statistically significant difference was also found (P = 0.038) (Figure 1B). Serum levels of B domain containing Tn-C were significantly higher in patients with arterial hypertension and LVH than in normal volunteers (P = 0.020). Within the group of patients with LVH, serum concentrations of B domain containing Tn-C were significantly higher in patients with EH than in patients with CH (P = 0.003) (Figure 1A). Serum levels of C domain containing Tn-C were distinctly higher in patients with arterial hypertension and LVH than in normal volunteers (P = 0.042). Patients with EH had higher serum concentrations than patients with CH but the difference between the groups was not significant (P = 0.138) (Figure 1B).
Serum levels of matrix metalloproteinase-9
For MMP-9, statistically significant differences in serum concentrations between the three groups (normal volunteers, patients with CH, and patients with EH) were detected (P = 0.003) (Figure 1C). Serum concentrations of MMP-9 were significantly higher in patients with arterial hypertension and LVH (EH + CH) than in the normal volunteers (P = 0.013). Patients with CH had higher serum levels of MMP-9 than patients with EH (P = 0.028) (Figure 1C).
Serum levels of tissue inhibitors of matrix metalloproteinase-1, −2, and −4
For TIMP-1, −2, and −4, no significant differences in the serum concentrations between the three groups (normal volunteers, patients with CH, and patients with EH) were detected (TIMP-1, P = 0.169; TIMP-2, P = 0.074; TIMP-4, P = 0.275) (Figure 1D–F). In patients with arterial hypertension and LVH (EH + CH), serum concentrations of TIMP-1, −2, and −4 were higher than in the normal volunteers. For TIMP-2 but not for TIMP-1 and −4, these differences showed statistical significance (TIMP-1, P = 0.059; TIMP-2, P = 0.043; TIMP-4, P = 0.163) (Figure 1D–F). When comparing EH and CH in the group of patients with arterial hypertension and LVH, for all three proteases (TIMP-1, −2, and −4) no significant differences in serum levels could be detected (TIMP-1, P = 0.967; TIMP-2, P = 0.313; TIMP-4, P = 0.414). Comparing the group of patients with CH and the normal volunteers, there were borderline statistically significant differences in the serum concentrations of TIMP-1 (P = 0.062) and TIMP-2 (0.055) but not of TIMP-4 (P = 0.238) (Figure 1D–F). Comparing the group of patients with EH and the normal volunteers, significant differences were observed for TIMP-2 (P = 0.036) but not for TIMP-1 (P = 0.082) or TIMP-4 (P = 0.139) (Figure 1E and F).
Serum levels of B domain containing tenascin-C might discriminate between concentric and eccentric left ventricular hypertrophy
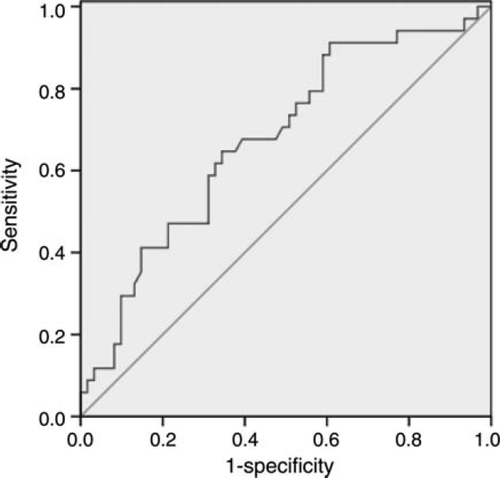
Since B domain containing Tn-C was found to be significantly higher in patients with EH vs. CH, we performed an ROC curve analysis to identify a cut-off level that might discriminate between the two forms of LVH in patients with arterial hypertension. On the basis of these results, we propose a cut-off value of ~900 ng/mL to discriminate CH (<900 ng/mL) from EH (>900 ng/mL) with a sensitivity of 65% and a specificity of 62% (Figure 2).
Discussion
B and C domains containing Tn-C serum levels were significantly higher in patients with hypertensive heart disease than in healthy controls. Patients with EH had significantly higher serum levels of B but not C domain containing Tn-C than those with CH. These data suggest a special role for the B domain of Tn-C within the process of myocardial remodelling leading to EH. Accordingly, re-expression of Tn-C variants has been demonstrated in a number of different heart diseases.18–20,23,33 Using ROC curve analysis, we propose a cut-off value of ~900 ng/mL with a sensitivity of 65% and a specificity of 62% to discriminate concentric from eccentric hypertrophy. To possibly reach higher sensitivity or specificity levels, this cut-off value needs to be evaluated in a larger number of patients.
The biological mechanisms which lead to increased serum levels of B domain Tn-C in hypertension-associated EH are not fully understood. On the basis that Tn-C is highly expressed during embryonic development of the heart and the functional importance for cardiomyocyte differentiation has been demonstrated in mouse models, re-expression under pathological conditions suggests a crucial regulatory role during myocardial remodelling.17 Accordingly, Tn-C re-expression has been described in heart diseases like myocarditis and myocardial injury.18,20 The differential analysis of serum levels of B and C domains containing Tn-C, especially in hypertensive heart disease, has never been investigated before; but is of interest due to their potential usability as diagnostic markers or if applicable as therapeutic target molecules.
For MMP-9, significant differences in serum levels were demonstrated between healthy volunteers and hypertensive patients. Within the group of patients with LVH, there were significant differences in MMP-9 levels between the concentric vs. the eccentric form, with decreased levels in the eccentric group. Matrix metalloproteinase-9 is known to play an important role in hypertension-induced left ventricular remodelling and in congestive heart failure, which is reflected by the increased serum levels in these patients.13 Yan et al.31 reported a positive correlation between elevated MMP-9 serum levels and reduced LVEF in patients with heart failure. We also showed that in hypertensive patients with signs of LVH, serum levels of MMP-9 were significantly higher than in normal volunteers. Thus, we can partially confirm the data from Ahmed et al. who reported increased MMP-9 levels in hypertension-induced congestive heart failure. In contrast to our findings for B domain containing Tn-C, serum levels of MMP-9 in patients with EH were decreased compared with those with CH. In our view, MMP-9 might be useful as a serum marker for the development of LVH in hypertensive patients. This is supported by the fact that in healthy humans, a decrease in MMP-9 serum levels occurs with increasing age.34 Moreover, reverse ventricular remodelling following cardiac resynchronization therapy has been shown to be associated with decreased levels of MMP-9 and also Tn-C.35
We did not show any differences in serum levels of TIMP-1, −2, and −4 between normal volunteers and patients with arterial hypertension and LVH. In addition, we could not find any significant differences between the groups with CH vs. EH. Therefore, we suggest that an increased expression of TIMPs is important in the process of myocardial remodelling due to chronic hypertension but does not play a crucial role in the determination of which form of LVH occurs. For patients with congestive heart failure, changes in the expression levels of TIMP-1 have been reported, Yang et al.36 reported decreased levels, whereas George et al.37 showed increased TIMP-1 expression compared to normal controls. These inconsistent findings should be discussed in the context of age-dependent changes in TIMP expression. Thus, in an ageing population free of clinically significant cardiovascular disease, an increase in serum levels of TIMP-1, −2, and −4 could be shown with increasing subject age.35
Study limitations
There are limitations to this study. First, measurement of serum levels of ECM components does not provide evidence of myocardial origin. To minimize the impact of non-cardiac sources, we excluded patients with diseases clearly associated with ECM remodelling. Measurements in coronary sinus blood compared with peripheral blood might be useful for determining ECM components of myocardial origin. Furthermore, analysis of protein expression in myocardial tissue of hypertensive patients with CH vs. EH should be the object of further studies. Secondly, the influence of antihypertensive treatment with particular regard to the renin–angiotensin–aldosterone system has not been investigated in our study.
Conclusions
In summary, it can be postulated, first, that alterations in the serum levels of MMP-9 and TIMP-1, −2, and −4 as well as the differential re-expression of Tn-C splice variants are associated with myocardial ECM remodelling in LVH. Secondly, B domain containing Tn-C, and C domain containing Tn-C, MMP-9, and TIMP-2 might play important roles in the process of LVH-associated ECM remodelling. Thirdly, the B domain containing Tn-C may be considered as a useful diagnostic serum marker for discrimination of the two forms of LVH in hypertensive patients or as a potential new therapeutic target molecule.
Acknowledgements
The authors would like to thank Mrs Annett Schmidt for excellent technical assistance.
Funding
This research received funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreement no. Health-F2–2008–201342 (ADAMANT).
Conflict of interest: none declared.




