Pentraxin-3 and the Right Ventricle: The Multi-Ethnic Study of Atherosclerosis–Right Ventricle Study
Abstract
Pentraxin-3 (PTX3) is a protein mediator of innate immunity that is elevated in the setting of left heart disease and pulmonary arterial hypertension. The relationship between PTX3 and right ventricular (RV) structure and function is not known. We included men and women with magnetic resonance imaging assessment of RV structure and function and measurement of PTX3 from the Multi-Ethnic Study of Atherosclerosis, a study of individuals free of clinical cardiovascular disease. Multivariable linear regression estimated associations between PTX3 protein levels and RV measures after adjusting for demographic characteristics, anthropometrics, smoking status, diabetes mellitus, hypertension, and corresponding left ventricular (LV) parameters. Instrumental variable analysis exploiting Mendelian randomization was attempted using two-stage least squares regression. The study sample included 1,779 participants with available PTX3 levels, RV measures, and all covariables. Mean PTX3 level was 2.1 ng/mL. Higher PTX3 was independently associated with greater RV mass and larger RV end-diastolic volume with and without adjustment for the corresponding LV parameters or C-reactive protein (all P <. 05). There was no association between PTX3 and RV ejection fraction or stroke volume. Single-nucleotide polymorphisms were not associated with PTX3 protein levels or RV measures after accounting for race. Instrumental variable analysis could not be reliably performed. Higher PTX3 protein levels were associated with greater RV mass and larger RV end-diastolic volume. These associations were independent of common cardiovascular risk factors and LV morphologic changes. Inflammation is associated with differences in the pulmonary circulation-RV axis in adults without clinical cardiovascular disease.
INTRODUCTION
Pentraxin-3 (PTX3) is a protein mediator of innate immunity produced by vascular smooth muscle cells, endothelial cells, and fibroblasts. PTX3 may dampen the immune response through interruption of the complement cascade, apoptotic clearance, and mitigation of atherosclerosis.1 PTX3 is found in atherosclerotic plaques, and increased circulating levels are seen in diastolic dysfunction, metabolic syndrome, and myocarditis.2-5 High PTX3 levels also suggest poor prognosis in chronic heart failure and other diseases.6-8
PTX3 may also be important in pulmonary vascular disease and right ventricular (RV) dysfunction. High PTX3 levels are reported in patients with pulmonary hypertension,9 and local control of inflammation is hypothesized to influence RV adaptation.10 Furthermore, specific PTX3 genotypes are associated with increased risk for primary graft dysfunction after lung transplant, for which pulmonary hypertension is also a known risk factor.11,12 The relationship between PTX3 and RV structure and function has not been studied.
We examined the relationship of PTX3 with magnetic resonance imaging (MRI) measures of RV structure and function in a multiethnic cohort of adults free of clinical cardiovascular disease. We hypothesized that higher PTX3 protein levels would be independently associated with greater RV mass, larger end-diastolic volume, and reduced RV ejection fraction.
METHODS
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter prospective cohort study designed to investigate the prevalence, correlates, and progression of subclinical cardiovascular disease in whites, African Americans, Hispanics, and Chinese Americans.13 In 2000–2002, MESA recruited 6,814 men and women aged 45–84 years old from 6 US communities: Forsyth County, NC; northern Manhattan and the Bronx, NY; Baltimore City and Baltimore County, MD; St. Paul, MN; Chicago, IL; and Los Angeles, CA. Exclusion criteria included clinical cardiovascular disease (physician-diagnosed heart attack, stroke, transient ischemic attack, heart failure, angina, current atrial fibrillation, and any cardiovascular procedure), weight greater than 136 kg (300 lb), pregnancy, or impediment to long-term participation. The institutional review boards of all participating institutions approved the protocols of MESA and all studies described herein. The MESA-Right Ventricle Study, which includes this investigation, is an ancillary study with funding to interpret approximately 4,200 cardiac MRIs for RV morphology in MESA participants at the baseline examination.
Cardiac MRI measures
The cardiac MRI protocol has been previously described.14 All imaging was performed on 1.5-tesla magnets. Imaging consisted of fast-gradient echo cine images with temporal resolution of 50 ms or less. Cardiac MRI examination findings were analyzed at the reading center at Johns Hopkins University in Baltimore, Maryland. Image analysis was performed by two independent analysts on Windows workstations using QMASS software (Medis, Leiden, the Netherlands). Images were magnified to 250%, contrast and brightness were set to 55, and window width and level were set with auto function in QMASS.
Methods for interpretation of left ventricle (LV) and RV parameters have been reported elsewhere.15,16 Endocardial and epicardial borders of the RV were manually traced on MRI short-axis cine images at end-systole and end-diastole. Full visualization of the correct placement of RV contours relied on evaluation of cine images to determine the demarcation between the right atrium and the RV. Contours were modified at basal slices of the heart by careful identification of the tricuspid valve to exclude the right atrium and to avoid overestimation of the volumes. The outflow tract was included in RV volume. Papillary muscles and trabeculae were included in RV volumes and excluded from RV mass, as is commonly done for LV mass.17,18
RV end-systolic volume and RV end-diastolic volume (RVEDV) were calculated using Simpson's rule by summation of areas on each slice multiplied by the sum of slice thickness and image gap. RV mass was determined at the end-diastole phase as the difference between end-diastolic epicardial and endocardial volumes of the RV free wall multiplied by the specific gravity of the heart (1.05 g/mL). RV stroke volume (SV) was calculated by subtracting RV end-systolic volume from the RVEDV. RVEF was calculated by dividing RVSV by RVEDV.
Our protocol included random blinded rereads by the same reader. The intrareader intraclass correlation coefficient for RV mass was 0.94 (229 scans); for RVEDV, it was 0.99 (230 scans); and for RVEF, it was 0.89 (230 scans). In addition, blinded rereads by a second reader were performed. The interreader intraclass correlation coefficients on 240 scans for RV mass, RVEDV, and RVEF were 0.89, 0.96, and 0.80, respectively.
Biomarkers and covariables
Fasting blood samples were obtained, processed, and stored using standardized procedures.19 PTX3 was measured as part of another ancillary study involving 2,880 MESA participants. Participants underwent novel biomarker testing and were selected to achieve balanced representation from all four race/ethnic groups to allow sufficient power to conduct subgroup analyses in each racial/ethnic group. PTX3 was measured at the Laboratory for Clinical Biochemistry Research at University of Vermont (Burlington) using a sandwich enzyme-linked immunosorbent assay (PTX3 [human] detection set; Alexis Biochemicals, San Diego, CA). The analytic coefficient of variation was 10.2%.
Genotyping was attempted in a random sample of participants in MESA who gave consent for genetic analysis and from whom DNA was extracted from peripheral leukocytes using a commercially available isolation platform (Puregene; Minneapolis, MN).20 Genotyping was performed by Illumina Genotyping Services (San Diego, CA) using the GoldenGate Assay. After removal of failed single-nucleotide polymorphisms (SNPs) and samples, the genotyping call rate was 99.93%. The human PTX3 gene, located on chromosome 3q25.32, consists of 3 exons separated by 2 introns and covering 7 kilobases.21 Several SNPs have been described in the PTX3 gene, including SNPs with potential functional significance. The GAG haplotype for sites rs2305619, rs3816527, and rs1840680, in particular, has been associated with increased PTX3 protein levels,22 a reduced risk of tuberculosis,23 a reduced risk of Pseudomonas species colonization in cystic fibrosis,21 and improved female fertility.22 There were 6 available SNPs in the PTX3 gene locus considered for analysis.
Other covariables, including race/ethnicity, height, weight, presence of hypertension or diabetes mellitus, C-reactive protein (CRP), smoking status, spirometry, and emphysema, were measured as described elsewhere (see appendix, available online).24
Statistical analysis
We used linear regression to characterize the relationship between PTX3 and RV parameters. PTX3 was log transformed to achieve normality. All models were adjusted for height and weight, so it was not necessary to index RV parameters to account for differences in body size. Generalized additive models were used to assess possible nonlinearity. Covariables were chosen on the basis of known associations with ventricular size, heart disease, and comorbidities. In limited models, we adjusted for age, sex, race/ethnicity, height, and weight. In fully adjusted models, we included educational level, income, smoking status, hypertension, diabetes mellitus, cholesterol, and impaired glucose tolerance. We adjusted for LV parameters to evaluate for independence from LV abnormalities (e.g., increased LV mass causing pulmonary venous hypertension leading to increased RV mass) and to better account for differences in body size. Because RVSV and LVSV are interdependent, we adjusted for LV mass in this case. We adjusted for both structural and functional lung disease using percentage emphysema on chest computed tomography, forced expiratory volume at 1 second, and forced vital capacity, when available (n = 1,360). Because of theoretical antagonism between CRP and PTX3, we performed additional analyses adjusted for CRP level (n = 1,772).
In participants with available SNPs in the PTX3 gene locus (n = 1,582), departures from Hardy-Weinberg equilibrium were assessed using the Fisher exact test. Multiple linear regression was used to characterize the relationship between candidate genotypes/haplotypes and PTX3 protein levels or RV measures in models with and without adjustment for race and principal components.25 Because the true nature of inheritance is unknown, both additive and dominant models were assessed. Instrumental variable analysis exploiting Mendelian randomization was attempted using two-stage least squares regression.26
Statistical significance was defined as P <. 05 for all nongenetic analyses. For genetic analyses, we used a Bonferroni correction for 12 comparisons so that P <. 004 was considered significant. Analyses were performed using STATA 12.0 (StataCorp, College Station, TX). Results of genetic analyses were confirmed using the Golden Helix software package (Bozeman, MT).
RESULTS
There were 6,814 men and women enrolled in MESA (Fig. 1), of whom 5,098 underwent cardiac MRI and 5,004 (98%) had interpretable examinations for the LV. The RV was successfully interpreted in 4,204 participants (95% of 4,424 attempted). PTX3 was measured in 1,856 of these participants under another ancillary study. Seventy-seven participants were excluded for missing covariables, leaving 1,779 in the study sample. Table 1 shows characteristics of the study sample compared with those excluded. The mean age of the study sample was 60.7 years, and 46.1% of the participants were men. As compared with MESA participants not included in the analysis, the study sample had a larger proportion of Chinese race and fewer whites, which reflects oversampling of this racial group in the PTX3 ancillary study design. Formal statistical testing is not appropriate for such comparisons, which are descriptive rather than inferential. Mean RV mass in the study sample was 20.9 ± 4.5 g, mean RVEDV was 121.3 ± 30.5 mL, mean RVSV was 84.6 ± 20.2 mL, and mean RVEF was 70.3% ± 6.6%. Mean PTX3 level was 2.1 ng/mL, with an intraquartile range of 1.4–2.5 ng/mL.
Study sample. LV: left ventricle; MESA: Multi-Ethnic Study of Atherosclerosis; MRI: magnetic resonance imaging; PTX3: pentraxin-3; RV: right ventricle.
| Variable | Study sample (n = 1,779) | Excluded (n = 5,035) |
|---|---|---|
| Age, mean ± SD, years | 60.7 ± 10.0 | 62.7 ± 10.3 |
| Male sex | 46.1 | 47.5 |
| Race/ethnicity | ||
| White | 26.6 | 42.7 |
| Chinese American | 26.5 | 6.6 |
| African American | 23.5 | 29.3 |
| Hispanic | 23.4 | 21.4 |
| Educational attainment | ||
| No high school degree | 19.2 | 17.6 |
| High school degree | 17.6 | 18.4 |
| Some college | 14.4 | 17.0 |
| Bachelor's degree | 18.1 | 17.0 |
| Higher than bachelor's degree | 17.9 | 18.1 |
| Height, mean ± SD, cm | 165.1 ± 9.8 | 166.8 ± 10.1 |
| Weight, mean ± SD, kg | 74.5 ± 16.3 | 80.1 ± 17.4 |
| Body mass index, mean ± SD | 27.2 ± 5.0 | 28.7 ± 5.6 |
| Cigarette smoking status | ||
| Never | 57.2 | 47.9 |
| Former | 30.0 | 38.9 |
| Current | 12.8 | 13.2 |
| Hypertension | 40.7 | 46.5 |
| Systolic blood pressure, mean ± SD, mmHg | 124.6 ± 21.0 | 127.3 ± 21.6 |
| Diastolic blood pressure, mean ± SD, mmHg | 71.7 ± 10.0 | 72.0 ± 10.3 |
| Diabetes mellitus | 12.9 | 13.8 |
| PTX3, mean ± SD, ng/mL | 2.1 ± 1.2 | 2.2 ± 1.6a |
- Note: Data are % of patients, unless otherwise indicated. Body mass index was defined as weight in kilograms divided by the square of height in meters. PTX3: pentraxin-3; SD: standard deviation.
- a A total of 1,007 participants with measured PTX3 were not included in the study sample because of unavailable magnetic resonance imaging or missing covariables.
Higher PTX3 was associated with greater RV mass (0.3-g increase per log increase in PTX3, P =. 04). This relationship was not altered by adjustment for cardiovascular risk factors (P =. 03) or LV mass (P =. 02; Table 2; Fig. 2). Further adjustment for lung structure and function did not alter the relationship between PTX3 and RV mass in the 1,360 participants with these measures (0.4-g increase per log increase in PTX3, P =. 03). Adjustment for CRP did not change the relationship between PTX3 and RV mass (0.4-g increase per log increase in PTX3, P =. 02; Table 3).
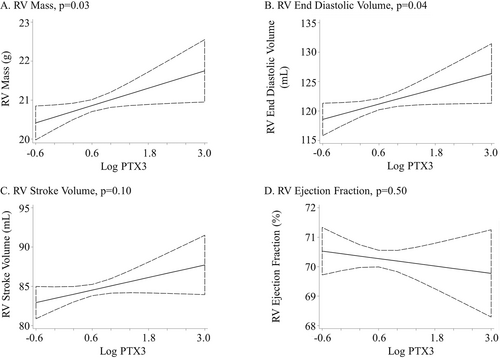
Association between the log of pentraxin-3 (PTX3) protein level and right ventricle (RV) parameters. Linear regression adjusted for age, sex, race/ethnicity, education, income, height, weight, smoking status, presence of hypertension or diabetes mellitus, cholesterol, and impaired glucose tolerance.
| Per log increase in PTX3 | |||
|---|---|---|---|
| Variable | Coefficient | 95% CI | P |
| RV mass, g | |||
| Limited model | 0.3 | 0.0–0.7 | .04 |
| Adjusted model | 0.4 | 0.0–0.7 | .03 |
| Adjusted plus LV mass | 0.4 | 0.1–0.7 | .02 |
| RVEDV, mL | |||
| Limited model | 1.8 | –0.3 to 3.9 | .09 |
| Adjusted model | 2.2 | 0.1–4.3 | .04 |
| Adjusted plus LVEDV | 2.3 | 0.8–3.8 | .003 |
| RVSV, mL | |||
| Limited model | 1.0 | –0.5 to 2.6 | .19 |
| Adjusted model | 1.3 | –0.2 to 2.9 | .10 |
| Adjusted plus LV mass | 1.3 | –0.2 to 2.8 | .08 |
| RVEF, % | |||
| Limited model | –0.2 | –0.9 to 0.4 | .44 |
| Adjusted model | –0.2 | –0.8 to 0.4 | .50 |
| Adjusted plus LVEF | –0.2 | –0.7 to 0.4 | .55 |
- Note: The limited model includes age, sex, race/ethnicity, height, and weight. The adjusted model includes the limited model plus education, income, smoking status, presence of hypertension or diabetes mellitus, cholesterol level, and impaired glucose tolerance. CI: confidence interval; EDV: end-diastolic volume; EF: ejection fraction; LV: left ventricle; SV: stroke volume.
| Per log increase in PTX3 | |||
|---|---|---|---|
| Variable | Coefficient | 95% CI | P |
| Adjusted for lung structure and function (n = 1,360) | |||
| RV mass, g | 0.4 | 0.0–0.8 | .03 |
| RVEDV, mL | 2.0 | –0.3 to 4.4 | .09 |
| RVSV, mL | 1.1 | –0.7 to 2.8 | .23 |
| RVEF, % | –0.3 | –1.0 to 0.4 | .39 |
| Adjusted for C-reactive protein (n = 1,772) | |||
| RV mass, g | 0.4 | 0.1–0.7 | .02 |
| RVEDV, mL | 2.4 | 0.3–4.5 | .03 |
| RVSV, mL | 1.4 | –0.1 to 2.9 | .07 |
| RVEF, % | –0.2 | –0.9 to 0.4 | .45 |
- Note: Lung structure and function adjustment included the adjusted model plus forced expiratory volume at 1 second, forced vital capacity, and percentage emphysema on chest computed tomography. C-reactive protein adjustment included the adjusted model plus C-reactive protein level. CI: confidence interval; EDV: end-diastolic volume; EF: ejection fraction; LV: left ventricle; RV: right ventricle; SV: stroke volume.
Higher PTX3 protein level had a borderline association with larger RVEDV after limited adjustment (1.8-mL increase per log increase in PTX3, P =. 09). Full adjustment accounting for confounding by cardiovascular risk factors strengthened the relationship (P =. 04), as did additional adjustment for LVEDV (P =. 003; Table 2; Fig. 2). Additional adjustment for lung structure and function attenuated the relationship (2.0-mL increase per log increase in PTX3, P =. 09), and additional adjustment for CRP did not change the relationship between PTX3 and RVEDV (2.4-mL increase per log increase in PTX3, P =. 03; Table 3).
PTX3 protein level was not associated with RVSV or RVEF (Table 2; Fig. 2). Adjustment for cardiovascular risk factors, LV mass or ejection fraction, CRP level, or lung structure and function did not alter these results (Table 2, 3).
In exploratory analyses, there was no effect modification by age, sex, race/ethnicity, or LV parameters. No significant nonlinearity was detected using generalized additive models (all P >. 2).
Of 1,779 participants, 1,582 had genotypes available for analysis (86.4%). Of available PTX3 SNPs, rs3845978, rs9289983, and rs2614 were not in Hardy-Weinberg equilibrium (Table S1). When stratified by race/ethnicity, only rs2614 was not in Hardy-Weinberg equilibrium (Table S2). None of the 5 candidate genes with a minor allele frequency greater than 5% (rs3845978, rs2305619, rs2120243, rs1456099, and rs9289983) were associated with PTX3 protein levels, RV mass, or RVEDV after adjustment for principal components (Table 4). Additional adjustment for race/ethnicity did not change these results. There was no significant interaction with race/ethnicity. Exploratory relationships stratified by race/ethnicity approached but did not achieve significance for some evaluated SNPs (Tables S3, S4). The first-stage F statistic in all instrumental variable analyses was less than 10, which suggests a poor instrument. The weak association between selected SNPs and PTX3 protein level precluded meaningful analysis using this technique (Table S5).27
| Genetic model (P values) | ||||||
|---|---|---|---|---|---|---|
| Association with PTX3 protein level | Association with RV mass | Association with RVEDV | ||||
| Variable | Additive | Dominant | Additive | Dominant | Additive | Dominant |
| rs3845978 | ||||||
| Unadjusted | .03 | .07 | .007 | .02 | .03 | .08 |
| Adjusted | .24 | .46 | .23 | .38 | .48 | .76 |
| rs2305619 | ||||||
| Unadjusted | .57 | .37 | .008 | .03 | .005 | .01 |
| Adjusted | .85 | .83 | .76 | .80 | .53 | .46 |
| rs2120243 | ||||||
| Unadjusted | .75 | .70 | .11 | .10 | .07 | .04 |
| Adjusted | .68 | .83 | .95 | .82 | .75 | .44 |
| rs1456099 | ||||||
| Unadjusted | .49 | .73 | .32 | .27 | .14 | .29 |
| Adjusted | .55 | .75 | .96 | .97 | .56 | .94 |
| rs9289983 | ||||||
| Unadjusted | .36 | .59 | .19 | .10 | .07 | .10 |
| Adjusted | .37 | .57 | .97 | .87 | .59 | .84 |
- Note: Adjusted model includes principal components.
DISCUSSION
We have shown that higher PTX3 protein levels are associated with greater RV mass and larger RVEDV in a multicity cohort of adults without clinical cardiovascular disease. The relationship with RV mass was not strongly affected by adjustment for differences in pulmonary structure and function or by differences in respective LV mass, which suggests an RV-specific relationship. MESA participants had a 0.3-g (1.4%) increase in RV mass per log increase in PTX3. This is similar to the difference seen in LV mass (2.4%) in MESA participants with and without diabetes and may support the clinical and biological relevance of this association.28 In addition, RV hypertrophy in MESA is associated with a threefold higher risk for cardiovascular death or heart failure.29
Our findings suggest the importance of PTX3 in the pulmonary circulation–RV axis. An increasingly nuanced understanding of inflammation in the pulmonary circulation and RV is necessary as immunomodulators such as FK506 are considered as treatments for PAH.30 Inflammation is broadly implicated in pulmonary vascular disease, and recent work confirms that PTX3 is elevated in pulmonary arterial hypertension.9,31 Our findings may reflect subclinical pulmonary vascular disease with decreased ability to recruit the pulmonary vasculature, leading to increased afterload, RV hypertrophy, and dilation. The LV undergoes similar hypertrophy even in response to mild systemic hypertension; hypertrophy in the thin-walled RV may be more pronounced.32 Unlike systemic hypertension, however, small changes in the pulmonary vasculature typically do not result in increased resting pulmonary arterial pressure due to the large-flow volume reserve of the pulmonary vasculature.33
A direct relationship between PTX3 and RV structure independent of pulmonary vascular disease may also contribute to our findings. Myocardial inflammation can promote LV hypertrophy both in the presence and absence of pathologic afterload.34-36 The pathophysiologic role of inflammation in determining RV structure may explain the variable susceptibility to right heart failure that exists among patients with identical afterload.37
Although CRP and PTX3 plasma levels are both elevated in pulmonary hypertension, their relationship with the RV differs in adults without clinical cardiovascular disease. High levels of CRP are associated with lower RV mass and a smaller RVEDV, which is opposite to the greater mass and larger RVEDV that we currently describe with high levels of PTX3.9,38,39 Animal models have found that CRP potentiates and PTX3 dampens myocardial inflammation and that, in patients with heart failure, rosuvastatin decreases CRP levels while increasing PTX3 protein levels.7,40-42 The different relationships of CRP and PTX3 to RV structure cautiously support this paradigm of distinct and opposing biologic roles for CRP and PTX3 in cardiac pathophysiology and expand this paradigm to the RV.
Finally, we evaluated several SNPs in the PTX3 gene locus and found no association with PTX3 protein level or RV structure after accounting for race/racial stratification. Previous analyses of similar PTX3 genotypes have shown increased risk for Pseudomonas in patients with cystic fibrosis and increased PTX3 protein levels and primary graft dysfunction in patients who undergo lung transplant.11,21 Both cystic fibrosis and lung transplant are pathologically inflamed states. The strong relationship of PTX3 genotype to aspects of these diseases, not seen in our largely healthy cohort, may suggest that the studied PTX3 genotypes have a greater effect on PTX3 levels only in the setting of robust inflammation. The lack of association with previously reported genotypes may therefore reflect the lack of pathologic inflammation in the relatively healthy MESA cohort and the relevance of these genotypes only in the setting of such inflammation.
This study has limitations. As is possible in all observational studies, residual or unmeasured confounding could account for the results. Our study was cross-sectional, and causality cannot be assessed. Unfortunately, measurement of invasive pulmonary hemodynamics, which may have informed the mechanism underlying our results, was not feasible in this large study of community-dwelling participants free of cardiovascular disease. Finally, this cohort is reasonably healthy, leading to relatively small effect estimates. However, such estimates are similar in scale to other clinically significant associations in MESA.28
Summary. Higher PTX3 levels are associated with greater RV mass and larger RVEDV. These relationships are independent of left-sided cardiovascular disease. This study contributes to the understanding of inflammation in the pulmonary circulation-RV axis at a time when immunomodulators are being considered for use in pulmonary hypertension.
ACKNOWLEDGMENTS
This manuscript was reviewed by the Multi-Ethnic Study of Atherosclerosis (MESA) investigators for scientific content and consistency of data interpretation with previous MESA publications, and significant comments were incorporated before submission for publication. We thank the other investigators, staff, and participants of the MESA and MESA-Lung Studies for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This work was presented in part as an abstract at the American Thoracic Society Meeting (Philadelphia; May 2013).
Source of Support
The National Institutes of Health supported this work (RC1-HL100543, R01-HL086719, K24-103844, R01-HL077612, N01-HC95159 through HC95165, and N01-HC95169). This publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR000421.
Conflict of Interest
None declared.




