A Comparison of Three Anatomical Structures for Estimating Age in a Slow-Growing Subarctic Population of Lake Whitefish
Abstract
It has been well documented in previous research that otoliths are the preferred hard structure for estimating age in coregonids. However, the slower growth due to short growing seasons experienced by populations in the subarctic region of the boreal zone may alter the utility of alternative nonlethal structures for estimating age. We compared the three most commonly used age estimation structures (otoliths, pectoral fin rays, and scales) for Lake Whitefish Coregonus clupeaformis in a northern (above 50°N) population from Great Slave Lake. This study provides new perspectives regarding the use of different aging structures with fish populations typical of subarctic and arctic environments. Results of ANOVA showed that reader confidence, within-reader precision, and the age estimates themselves were all affected by age structure; reader confidence also varied with age-class. Reader confidence was highest for age estimates from otoliths, followed by pectoral fin rays and then scales. Similarly, within-reader precision (as measured by CVs) was highest for age estimates from otoliths, followed by scales and then pectoral fin ray sections. Pairwise comparisons between age estimates from otoliths and those from scales or pectoral fin rays indicated no significant differences when fish were younger than 10 years (scales) or 12 years (fin rays), suggesting that these nonlethal structures could be conservatively used to reliably estimate ages of younger (<10 years) and smaller (≤300 mm FL) Lake Whitefish. Of particular significance are the findings that (1) divergence between scale and otolith age estimates is delayed by 5–6 years relative to more southerly populations; and (2) in contrast to examples from southern populations, fin rays do not offer a suitable nonlethal alternative for estimating ages of older Lake Whitefish (>11 years).
Received March 4, 2014; accepted December 3, 2014
Determination of fish age is a critical component of understanding fish population dynamics, as biological parameters of growth, mortality, and recruitment have been shown to be age dependent (Quinn and Deriso 1999). These demographic and biological characteristics are also impacted by variable population density, prey availability, and exploitation (Ricker 1975; Beamish and McFarlane 1983; Weatherley and Gill 1987). Age information forms the basis of age-structured fish population assessments and the subsequent development of management decisions to sustainably maintain the commercial, recreational, and aboriginal subsistence use of a fisheries resource (Ricker 1975; Quinn and Deriso 1999). Aging errors associated with inaccurate age estimation can result in a misunderstanding of fish population dynamics and can lead to inappropriate management recommendations (Beamish and McFarlane 2000; Bertignac and de Pontual 2007).
Estimates of fish age and growth are conventionally based on annual marks in scales, fin rays, and otoliths, taking into account variations in behavioral and physiological processes (Simkiss 1974; Brett 1979; Maceina et al. 2007). Scales are most commonly used for esocids, centrarchids, and moronids because their removal is easy and nonlethal and causes minimal stress to the fish (Maceina et al. 2007). However, scales from long-lived salmonid species, such as Lake Trout Salvelinus namaycush (Schram and Fabrizio 1998), Bull Trout Salvelinus confluentus (Zymonas and McMahon 2009), and Inconnu Stenodus leucichthys (Howland et al. 2004), are difficult to interpret, resulting in significantly lower age estimates, confidence, and agreement than are observed with otoliths and fin rays (Ihde and Chittenden 2002). In some cases, bony structures (e.g., pelvic fin rays) have been favored as an alternative nonlethal structure for these long-lived species (e.g., Inconnu; Howland et al. 2004) since the removal of fin rays does not require sacrificing the fish, whereas the removal of otoliths does. As age estimation techniques have advanced, multiple-structure comparisons for age estimation have allowed for selection of the most suitable structure(s) for a particular species or population (e.g., Howland et al. 2004; Zymonas and McMahon 2009; Buckmeier et al. 2012).
The Lake Whitefish Coregonus clupeaformis is a commonly exploited coldwater salmonid that is extensively distributed in North American freshwaters from Atlantic coastal watersheds westward and northward across Canada and Alaska (Scott and Crossman 1998). Van Oosten (1923) first explored the applicability of scales in age estimation for known-age Lake Whitefish in aquaria. Since then, many age and growth studies of this species have been conducted with different aging structures, including scales (Mills and Beamish 1980; Barnes and Power 1984), otoliths (Barnes and Power 1984; Muir et al. 2008a, 2008b), and pectoral or pelvic fin rays (Mills and Beamish 1980; Barnes and Power 1984; Mills and Chalanchuk 2004; Muir et al. 2008b). Subsequent comparisons of multiple age estimation structures from Lake Whitefish have documented that (1) fin rays are more reliable than scales (Mills and Beamish 1980; Barnes and Power 1984; Muir et al. 2008b); (2) ages are considerably underestimated from the use of scales in comparison with otoliths (Barnes and Power 1984; Muir et al. 2008a, 2008b; Herbst and Marsden 2011); and (3) there are no significant differences between fin ray ages and otolith ages (Mills and Chalanchuk 2004; Muir et al. 2008b). Furthermore, in validating pelvic fin ray section ages for Lake Whitefish, Mills and Chalanchuk (2004) and others indirectly validated the otolith section method, as no significant difference was found between ages from fin rays and otolith sections. Since the break-and-burn method used with otoliths is considered to be the functional equivalent of the otolith section method (Campana et al. 2008), one can infer that the break-and-burn otolith method has also been indirectly validated for Lake Whitefish.
Although comparisons of aging structures have been conducted for southern populations of Lake Whitefish, there have been no comparable studies from more oligotrophic, subarctic boreal environments (i.e., greater than 50°N latitude), where fish are expected to have greater longevity and reduced growth (e.g., Power 1978; Morin et al. 1982). Annuli on aging structures from slower-growing northern stocks would be expected to begin condensing at a younger age, thus extending the area of annulus crowding in fish from long-lived populations. Previous studies have shown that annulus crowding at the edges of aging structures can lead to difficulties in interpretation and often to underestimation of ages, particularly in older fish (Power 1978; Mills and Beamish 1980). Scale and fin growth is directly related to somatic growth, whereas otoliths continue to grow as the fish ages (Beamish and McFarlane 1983); therefore, this edge interpretation problem is expected to be greater for scales and fin ray sections. Thus, we hypothesize that this problem could lead to greater underestimation of ages from scales and to potential underestimation of ages from fin ray sections beyond a certain age in Lake Whitefish from northern populations.
Underestimation of age has consequences for the assessment of fisheries when age-structured models are used to inform quota management, as all aspects of stock productivity and mortality require reliable estimates of age. For example, individual growth rates and estimated rates of increase in biomass are based on age determination. The age at sexual maturity is also important in consideration of recruitment overfishing and mortality estimates in general when partitioned between instantaneous fishing mortality and instantaneous natural mortality; mortality estimates for old-aged fish are especially influenced by the precision of estimates. Estimates of fishing mortality are used directly for determination of appropriate sustainable quotas.
Since the 1950s, Great Slave Lake (GSL) has sustained the largest commercial and aboriginal subsistence fisheries for Lake Whitefish in the Northwest Territories. The annual commercial harvest for Lake Whitefish alone peaked at about 1.7 million kg during the 1960s (Healey 1975) and diminished to around 416,000 kg in 2009–2010 (Zhu et al., unpublished data). Collection of scales for age estimation has constituted a routine activity in the monitoring of GSL Lake Whitefish fisheries production since 1972 (Read and Taptuna 2003). Based on findings from other age comparison studies, the use of scales as the primary aging structure for Lake Whitefish could result in significant underestimation of age (Mills and Beamish 1980; Barnes and Power 1984; Muir et al. 2008b). The objectives of this study were to compare age estimates obtained from three different aging structures (otoliths, pectoral fin rays, and scales) in GSL Lake Whitefish to (1) evaluate reader confidence in age interpretation; (2) evaluate the within-reader precision of age estimates; and (3) assess any observed patterns of discrepancy (e.g., age of divergence) in age estimates.
METHODS
Fish collections.—
Between July 10 and August 15, 2012, a total of 307 Lake Whitefish were captured with 32 bottom-set experimental gill nets. The nets were deployed at random locations in the shallower west basin of GSL (60°52.5–61°22.5′N, 113°35–115°5′W) using a depth-stratified sampling design. To include as many size-classes as possible, we utilized multimesh (clear monofilament) gill nets consisting of 10 panels with stretched mesh sizes ranging from 13 to 140 mm. Each net was deployed for 18–30 h to cover daytime and nighttime periods. All of the captured Lake Whitefish were sacrificed and then measured for FL (mm) and weight (g). Several scales (10–20) were removed from an area of the body ventral to the anterior edge of the dorsal fin and above the lateral line. One or two left leading pectoral fin rays were cut off as close to the body as possible by using bone cutters. Sagittal otoliths were extracted by use of bone cutters and forceps. All structures were deposited into labeled scale envelopes and were air-dried.
Preparation of anatomical structures.—
To prepare scales for reading, four to five nonresorptive scales were placed in a consistent orientation with the external surface facing up on a clean acetate slide. The slide was then passed through an Ann Arbor roller press (Muir et al. 2008b). For age estimates, the acetate slide was placed impression side up in a Realist Vantage 5 microfiche reader equipped with a 43–51× magnifying lens. The pectoral fin ray samples were trimmed, set in ColdCure epoxy (Industrial Formulators of Canada), and cut into transverse cross-sections by using a Buehler Isomet low-speed saw (Lake Bluff, Illinois) equipped with a 10.16-cm × 0.012-mm diamond wafering blade. At least three consecutive sections (~0.35 mm thick) were cut; the first section comprised the most proximal portion of the fin ray. The sections were air-dried and then affixed to labeled slides in a sequential order by using Cytoseal 280 mounting medium and a cover slip. Pectoral fin ray sections were viewed under reflected light with a dissecting microscope (20–80×). Otoliths were processed by applying a modified version of the break-and-burn technique of Christensen (1964) and Power (1978), referred to here as the “grind-and-bake” technique. On the otolith's distal surface, the nucleus was marked under a dissecting microscope using an ultra-fine-point Sharpie marker. The marked otolith was then placed on a paper towel and broken slightly anterior to the nucleus along the transverse plane by repeatedly scoring it with an X-Acto knife. The break surface of the posterior otolith piece was then ground down to the center of the nucleus, maintaining the transverse section plane, by using a Foredom bench lathe (Foredom Electric, Bethel, Connecticut) fitted with a cylindrical felt bob (medium hardness) that was wrapped with a strip of 30-m adhesive-backed lapping film (3M, St. Paul, Minnesota). After a number of otolith grinds had been prepared, they were baked for 10–30 s (depending on the size of the otolith piece) on a Fisher Thermix hot plate (Fisher Scientific) at the hottest setting (~400°C). The otolith was then observed by mounting it in modeling clay (ground surface up) in a small petri dish that was filled with enough water to cover the otolith half. Otoliths were viewed with a dissecting microscope and reflected light at 20–80× magnification.
Age estimation.—
Ages were estimated by three readers from the Department of Fisheries and Oceans Canada (DFO) and one reader from the Ontario Ministry of Natural Resources (OMNR). All readers had previous experience with the aging structures used and the methods employed. Without knowledge of fish size or capture location, each reader examined each aging structure three times; the exceptions were that scales were read two times by the reader from OMNR and one time by one of the DFO readers. All readers assigned a confidence index rating to each read to indicate their level of certainty. Ratings (as recommended by Casselman 1983) were as follows: 1 = very uncertain; 3 = uncertain; 5 = moderately confident; 7 = confident; and 9 = certain. The annuli on otoliths, pectoral fin rays, and scales were identified as described by Chilton and Beamish (1982) and Summerfelt and Hall (1987).
Statistical analyses.—
The CV was used to measure within-reader precision of age estimates from each aging structure (Campana 2001; Gregg et al. 2006; Muir et al. 2008b). The CV was averaged across samples to determine mean (±SD) age-class-specific and overall within-reader precision for each aging structure. The effects of aging structure type, fish age-class, and read (first, second, or third read) on estimates of reader confidence, within-reader precision, and age estimates were tested by using multifactorial or two-way ANOVA (Zar 2010), including interaction terms. One-way ANOVA was employed to (1) compare reader confidence, precision, and age estimates between hard aging structures at different ages; (2) analyze between-read variation among aging structures; and (3) compare age estimates between structures for fish at different FLs. Linear regression analysis was then used to assess the relationship between reader confidence and fish age-class, while Bonferroni multiple range tests were used to assess how age estimates varied with either aging structure or age-class (Zar 2010). Discrepancies in age estimates were compared graphically to detect the age at divergence between structures, and paired t-tests were used to assess the null hypothesis that the difference between age estimates was zero. Prior to analyses, the CV data were log transformed. For all statistical analyses, the Kolmogorov–Smirnov test of symmetry was applied to test the assumption of normality (Hoenig et al. 1995), and differences were considered significant based on an value of 0.05. Statistical analyses were conducted using R version 3.1 (R Development Core Team 2008) with the FSA package (Ogle 2014).
RESULTS
Among the Lake Whitefish captured (n = 307), FLs ranged from 125 to 510 mm and averaged 346 ± 4 mm (mean ± SE). Fish were excluded from this study if their scales were regenerated or their scale samples were absent (n = 7), otolith samples were cracked or absent (n = 5), or pectoral fin ray samples were broken or absent (n = 3). Thus, we were left with an effective sample size of 292 Lake Whitefish for subsequent analyses. Fish with otolith ages greater than 18 years were excluded from further statistical analysis to eliminate age-classes with small sample sizes (n < 5).
Reader Confidence
Based on multifactorial ANOVA, reader confidence index ratings differed significantly by age-class and age structure type, and the age-class × aging structure and read × aging structure interactions were significant (Table 1). Regression analysis showed that reader confidence index ratings decreased with increasing age-class for all aging structures (r = −0.23, df = 2,478, P < 0.0001); however, this relationship varied among aging structures, with scale readings showing the strongest decline in confidence with increasing age (r = −0.40, df = 834, P < 0.0001). For fish between age 2 and age 11, one-way ANOVA indicated no significant effect of age-class on reader confidence among aging structures (F2, 1,542 = 2.64, P = 0.07), with scale readings being associated with marginally higher confidence index values (mean ± SD = 5.88 ± 0.06) than otolith readings (5.86 ± 0.07) and pectoral fin ray readings (5.70 ± 0.06). Conversely, for age-12 and older fish, reader confidence index ratings differed significantly among structures (F2, 935 = 17.46, P < 0.0001) and was highest for otoliths (5.55 ± 0.06), followed by pectoral fins (5.40 ± 0.07) and scales (4.95 ± 0.09). Although the overall effect of read (first, second, or third) on reader confidence was marginally nonsignificant (P = 0.06), the reader confidence index ratings increased between the first and third reads for otoliths (r = 0.45, df = 280, P < 0.001) and pectoral fin rays (r = 0.44, df = 280, P < 0.001) and also increased between successive reads of scales (F2, 833 = 7.88, P < 0.001).
| Source | df | F | P |
|---|---|---|---|
| Reader confidence rating | |||
| Age-class | 16 | 11.23 | <0.0001 |
| Aging structure | 2 | 3.19 | <0.0001 |
| Read | 2 | 2.86 | 0.0574 |
| Age-class × aging structure | 32 | 3.43 | <0.0001 |
| Age-class × read | 32 | 1.16 | 0.2506 |
| Read × aging structure | 4 | 9.44 | <0.0001 |
| Age-class × aging structure × read | 62 | 1.42 | 0.0172 |
| Within-reader precision | |||
| Age-class | 16 | 0.63 | 0.8637 |
| Aging structure | 2 | 4.14 | 0.0163 |
| Age-class × aging structure | 32 | 1.55 | 0.0284 |
| Age estimates | |||
| Aging structure | 2 | 23.16 | <0.0001 |
| Read | 2 | 3.57 | 0.0284 |
| Aging structure × read | 4 | 0.93 | 0.4432 |
Within-Reader Precision
Based on two-way ANOVA, mean within-reader CV differed among age structure types (Table 1). Otolith age estimates had the lowest within-reader CV (mean ± SD = 6.19 ± 0.50) and were therefore most precise, followed by scales (7.27 ± 0.45) and pectoral fin rays (8.10 ± 0.43). Although the CV did not vary significantly among different age-classes, there was a significant interaction between age-class and aging structure. Comparisons of mean age-class-specific within-reader CV based on plots (Figure 1) and one-way ANOVA suggested that between age 2 and age 8, the CVs decreased for otolith age estimates (F6, 86 = 2.84, P = 0.01) and pectoral fin ray age estimates (F6, 86 = 2.99, P = 0.01); beyond age 8, the CVs remained relatively constant (otoliths: F9, 167 = 0.83, P = 0.64; pectoral fin rays: F9, 167 = 0.77, P = 0.67). In contrast, the within-reader CV of scale age estimates was similar across the entire range of ages (F16, 262 = 0.77, P = 0.72; Figure 1).
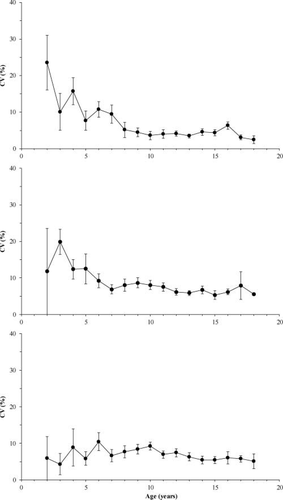
Mean (±SD) within-reader CV by age-class for ages estimated from the otoliths (prepared by the grind-and-bake technique; upper panel), sectioned pectoral fin rays (middle panel), and scales (lower panel) of Lake Whitefish sampled from Great Slave Lake.
Comparison of Age Estimates by Aging Structure and Read
Two-way ANOVA revealed that age estimates were significantly influenced by aging structure type and read and that the read × aging structure interaction was not significant (Table 1). Of the three aging structures, the greatest age range was generated by readings from pectoral fin rays (2–26 years), followed by otoliths (2–25 years) and scales (1–20 years; Table 2; Figure 2). However, the number of age-classes was lower from pectoral fin ray and scale readings (19 age-classes each) than from otolith readings (23 age-classes). Eighteen percent of otolith age estimates were greater than age 15, whereas only 14% of estimates from pectoral fin rays and 7% of estimates from scales were greater than age 15. As a result, paired t-tests showed that the mean age estimate from otolith readings (mean ± SE = 10.88 ± 0.16 years) was significantly greater than estimates from pectoral fin rays (9.82 ± 0.13 years; t = 5.17, df = 1,690, P < 0.0001) or scales (9.66 ± 0.13 years; t = 6.06, df = 1,690, P < 0.0001). The mean age estimates from pectoral fin rays and scales did not differ significantly (t = 1.05, df = 1,690, P = 0.29). Based on one-way ANOVA and examination of mean values, the age estimates differed and appeared to increase between subsequent reads for pectoral fin rays (F2, 833 = 4.85, P < 0.01), but age estimates did not vary in relation to read for otoliths (F2, 805 = 0.50, P = 0.60) or scales (F2, 832 = 0.34, P = 0.71).
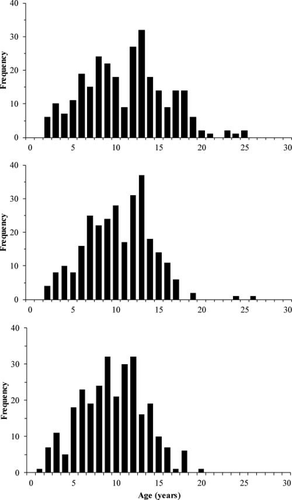
Comparison of age estimate frequencies obtained from readings of the otoliths (prepared by the grind-and-bake technique; upper panel), sectioned pectoral fin rays (middle panel), and scales (lower panel) of Lake Whitefish sampled from Great Slave Lake.
| Otolith age | Pectoral fin ray age | Scale age | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Read | Range | Mean | (SE) | Range | Mean | (SE) | Range | Mean | (SE) |
| Read 1 | 2–24 | 10.86 | (0.27) | 1–26 | 9.33 | (0.22) | 1–20 | 9.59 | (0.22) |
| Read 2 | 1–25 | 10.67 | (0.28) | 1–27 | 9.81 | (0.22) | 1–20 | 9.53 | (0.23) |
| Read 3 | 2–25 | 11.11 | (0.28) | 2–26 | 10.33 | (0.23) | 1–20 | 9.82 | (0.23) |
| Overall | 1–25 | 10.88 | (0.16) | 1–27 | 9.82 | (0.13) | 1–20 | 9.66 | (0.13) |
Pairwise comparisons of age estimates for these Lake Whitefish indicated that age differences varied with paired aging structures and assigned age-classes (Figure 3). When compared with otolith age estimates, the average age estimates from pectoral fin ray interpretation started to deviate from the 1:1 equivalence line after age 11. The average age estimates from scales deviated from the 1:1 line after age 9 when compared with otolith estimates and after age 11 when compared with pectoral fin ray estimates. Based on one-way ANOVA, otolith and pectoral fin ray age estimates were not significantly different up to age 11 (F1, 131 = 3.61, P = 0.06). Beyond age 11, the differences appeared to increase with age (F1, 136 = 48.38, P < 0.0001). When scale and otolith age estimates were compared, there was no significant difference up to age 9 (F1, 115 = 1.54, P = 0.22). Beyond age 9, there appeared to be a pronounced increase in the pairwise differences between estimates from scales and otoliths (F1, 163 = 73.43, P < 0.0001). No significant disagreement between scale and pectoral fin ray age estimates was detected up to age 12 (F1, 212 = 1.07, P = 0.30); however, differences were significant and appeared to increase with age thereafter (F1, 66 = 9.05, P < 0.005).
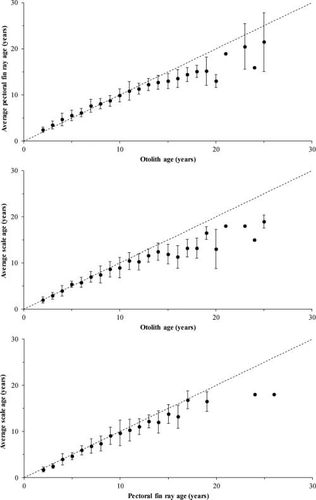
Age bias plots (mean age± SD) for the three aging structure pairings (dashed line = 1:1 equivalence line) for Lake Whitefish in Great Slave Lake.
Assessment of Age Discrepancies between Aging Structures in Relation to Lake Whitefish Length
The age discrepancies between aging structures were examined in relation to changes in FL for the GSL population (Figure 4). When pectoral fin ray readings were compared with otolith readings, the mean discrepancies were less than 1 year; based on one-way ANOVA, there were no significant differences in age estimates among fish with FLs less than 330 mm (F1, 116 = 2.66, P = 0.11; Figure 4a). For fish greater than 330 mm, there were significant differences among age estimates based on different structures (F1, 210 = 17.67, P < 0.001), and the differences appeared to increase with age. With the exception of the 260-mm length-class, the discrepancy between otolith and scale age estimates was±2 years, and the mean discrepancies were less than 1 year when FL was less than 310 mm. Age differences between otolith and scale readings did not differ for fish up to 300 mm FL (F1, 71 = 0.33, P = 0.57; Figure 4b). For fish greater than 300 mm, the age discrepancies between otolith and scale readings were significant (F1, 211 = 8.58, P < 0.005) and appeared to increase with age. No significant differences in age estimates were found between pectoral fin ray readings and scale readings over the entire fish size range (F1, 281 = 0.75, P = 0.95; Figure 4c); however, the variance between reads of these two structures was greater (more than ± 1 SD) at FLs beyond 310 mm.
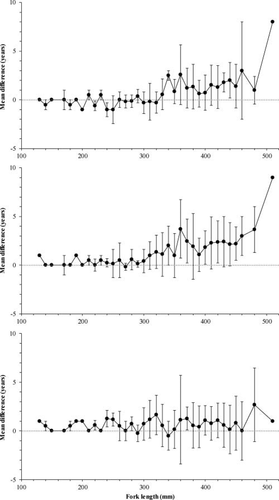
Relationship between fork length and mean (±SD) differences in age between readings from otoliths and pectoral fin rays (upper panel), otoliths and scales (middle panel), and pectoral fin rays and scales (lower panel) for Lake Whitefish from Great Slave Lake. The gray dotted line represents a zero difference over the fish length range.
DISCUSSION
Selection of the appropriate age estimation methods is dependent on the balance between the readability (ease of annulus interpretation) of aging structures and the consistency (repeatability) of the age estimates from those structures in addition to considerations of lethal versus nonlethal sampling (DeVries and Frie 1996; Ihde and Chittenden 2002; Herbst and Marsden 2011). In our study, readability based on reader confidence was found to be influenced by the interaction between age structure type and age-class. The older the fish, the lower was reader confidence, particularly in readings of scales. This result is similar to those from studies of Spotted Seatrout Cynoscion nebulosus in Chesapeake Bay (Ihde and Chittenden 2002) and Lake Whitefish in Lake Champlain (Herbst and Marsden 2011). Although scales from Lake Whitefish in our study were large enough to read, the readers reported that it was difficult to distinguish annuli once they became condensed at the outer edge, as is commonly the case in older fish. Annulus crowding likely contributed to the lower readability of scales—and, by extension, the lower confidence in scale readings—for fish beyond age 9.
With respect to the consistency of age estimates, we found that precision varied depending on the aging structure used. The highest precision (lowest CV) was found for readings from otoliths, suggesting that this structure is the most consistent. The suitability of otoliths for age estimation is further supported by the fact that otoliths do not show resorption and their growth is acellular rather than occurring by calcification (Secor et al. 1995). Otoliths are reported to be metabolically inert and thus do not reflect physiological changes that may occur throughout the life of the fish (Phelps et al. 2007).
In comparing age agreement and assignments among structures, the scale annuli appeared to be the most inconsistent, which led to lower agreement between reads, and scales produced the most variable results. This is consistent with the findings of other researchers (Mills and Beamish 1980; Muir et al. 2008a; Herbst and Marsden 2011). In particular, Mills and Beamish (1980) reported that agreement in estimated age between fin rays and scales was influenced by fish growth conditions. They found good agreement (fin ray and scale age estimates rarely differing by more than 1 year) for populations in environments favoring rapid growth, where estimated ages were seldom greater than 6 years. In contrast, precision was higher for fin ray age estimates than for scale age estimates in slow-growing populations with estimated fin ray ages that were often 8 years or greater.
In our comparison of the three different aging structures, we found that four age-classes were missing from demographic results when scales or pectoral fin rays were chosen for age estimates. Use of incorrect age composition data from less-reliable aging structures, especially scales, will lead to biases in assessing fish demographic parameters (e.g., growth [age-specific length], age-specific fishing mortality, and age-specific total mortality), estimating age-structured abundance and biomass, and establishing the minimum allowable fish size for fisheries regulation (Quinn and Deriso 1999; Campana 2001; Bertignac and de Pontual 2007).
Our results revealed that (1) in comparison with ages estimated from otoliths, scale ages can result in underestimates for fish older than 9 years; and (2) beyond age 9, the differences between otolith and scale ages increase with age. This represents a 5–6-year delay in age at divergence relative to that observed in studies of more southerly Lake Whitefish populations. For example, Barnes and Power (1984) found that scale age and otolith age estimates differed from ages 4–5 and beyond for Lake Whitefish in western Labrador. Similarly, in a study of Lake Whitefish in Lake Michigan, Muir et al. (2008b) reported that age estimates differed between scales and otoliths beginning at age 5 for the Baileys Harbor and Saugatuck stocks and beginning at age 8 for the Naubinway stock. The 5–6-year lag we observed was not expected; the lag may be due to the effects of different metabolic processes in slow-growing fish from this subarctic lake (Simkiss 1974; Healey 1975; Brett 1979). Indeed, the growth of Lake Whitefish in the present study was slower than that of populations in the more southerly located Laurentian Great Lakes (Cook et al. 2005; Muir et al. 2008a, 2008b) and is likely more typical of arctic and subarctic Lake Whitefish populations that experience a short growing season (Healey 1975; Morin et al. 1982).
When age estimates from pectoral fin ray and otolith sections were compared in more southerly stocks, no significant differences were found (Mills and Chalanchuk 2004; Muir et al. 2008b). Our study, however, indicated a divergence between age estimates from these two structures, with fin ray sections producing significant underestimation of age (on average, >1 year difference) beyond age 11, and this divergence increased with age. Such a result was not surprising given that fin ray growth is directly related to somatic growth, so greater difficulties in annulus interpretation are likely to occur with increased age. Although Mills and Chalanchuk (2004) validated the fin ray section method for all age-groups on a more southerly stock of Lake Whitefish, our data indicate that this method is not valid for older age-groups of the GSL stock and possibly other, more northerly stocks.
Differences between pectoral fin ray and scale age estimates were, on average, less than 1 year for fish up to age 9. Beyond this age, the pectoral fin ray age estimates were significantly greater than scale age estimates. Muir et al. (2008b) found that differences between age estimates from scales and pectoral fin rays began at age 5 for the Baileys Harbor stock (Lake Michigan) and at age 6 for the Naubinway and Saugatuck stocks. Again, this 4–5-year lag in age divergence may be attributable to different metabolic processes for fish further north and how these differences are manifested in the annular patterns on aging structures.
Based on our findings, we recommend otoliths as the best aging structure for use with GSL Lake Whitefish and other northern Lake Whitefish populations because of the otoliths’ higher readability and precision. However, our results indicate that comparable age estimates between the three aging structures can conservatively be obtained for GSL Lake Whitefish smaller than 300 mm FL; thus, we propose a size-specific use of alternative aging structures in the assessment of this fishery and similar northern fisheries. The ability to use alternative structures is an important consideration for fisheries scientists and managers, especially in cases where the collection of scales or fin rays without significantly damaging the fish is often the only option for sampling commercially caught fish. Sampling from the commercial catch offers a relatively low-cost option for demographic data collection. By balancing these considerations, we feel confident that both of the nonlethal aging structures (scales and pectoral fin rays) can be used to reliably estimate ages for GSL Lake Whitefish up to 300 mm FL without incurring significant age discrepancies relative to otolith readings.
This study provides new, relevant information to researchers and managers of the GSL Lake Whitefish fishery and yields new perspectives on the utility of different aging structures applicable to slow-growing Lake Whitefish populations in subarctic and arctic environments. Our study demonstrates that (1) in such populations, divergence between otolith and scale age estimates is delayed by 5–6 years relative to more southerly populations; and (2) in contrast to examples from southern Lake Whitefish populations, fin rays do not offer a suitable nonlethal alternative to otoliths for use with GSL Lake Whitefish older than age 11.
ACKNOWLEDGMENTS
We gratefully acknowledge the many people who helped with fish sample collections for this study, especially G. Low (Dehcho First Nations), S. Buckley (Hay River Metis Council), and D. Beaulieu, D. Lafferty, S. Biscaye, M. Edjericon, and S. Rymer (Deninu K'ue First Nations). We thank W. Irvine, A. Cook, and C. McDonald (OMNR) for assisting in preliminary analysis and sampling processing, and L. Vandenbyllaardt (DFO) for age estimation by reading otoliths. We also are grateful to J. Babaluk (retired DFO biologist) for his help in the method design and equipment selection for the otolith grind-and-bake technique. We greatly benefited from the information, logistic support, and text editing that were provided by C. Day, M. Treble, and M. Friesen (DFO). Critical reviews by three anonymous referees substantially improved the quality of the manuscript. This study was funded by the Northwest Territories Cumulative Impacts Monitoring Program and the DFO Aboriginal Fisheries Strategy.




